Abstract
Introduction: Though advances in therapy have improved outcomes for patients (pts) with multiple myeloma (MM) over the last decade, the disease remains largely incurable and optimal sequencing of therapies is not fully understood. Transplant remains a mainstay of MM treatment in the modern era. Clinical trial data generally informs regulatory approval of new therapies; however, real-world (RW) evidence can provide important insights into treatment patterns for MM and their impact on pt outcomes in a RW setting. The objective of this retrospective, observational cohort study was to better understand front-line treatments and outcomes among RW pts with newly diagnosed MM (NDMM), by stem cell transplant (SCT) status.
Methods: This study used the nationwide Flatiron Health electronic health record-derived, de-identified database of MM pts treated in the United States. During the study period of January 1, 2016, to January 31, 2022, pts whose front-line treatment was initiated after the study start date were included. We examined type of initial therapy received and pt outcomes by SCT in line 1 for the overall study population and, in post-hoc analyses, by pt subgroups classified according to age at start of front-line therapy, gender, race/ethnicity, International Staging System (ISS) stage at diagnosis, cytogenetic risk, presence of del(17p), presence of 1q21 amplification, renal function, and primary first-line treatment. RW progression-free survival (rwPFS) was defined as the time from the start of front-line therapy to the date of first progression event (informed by International Myeloma Working Group criteria and incorporating both abstracted M spike values and structured free light chain values) or death. RW overall survival (rwOS) was defined as the time from the start of front-line therapy to the date of death. Differences in rwPFS and rwOS by SCT were assessed using multivariable-adjusted Cox proportional hazards models, in which SCT was modeled as a time-varying covariate and adjustments were made for age, stage, cytogenetic risk, and other patient/disease characteristics.
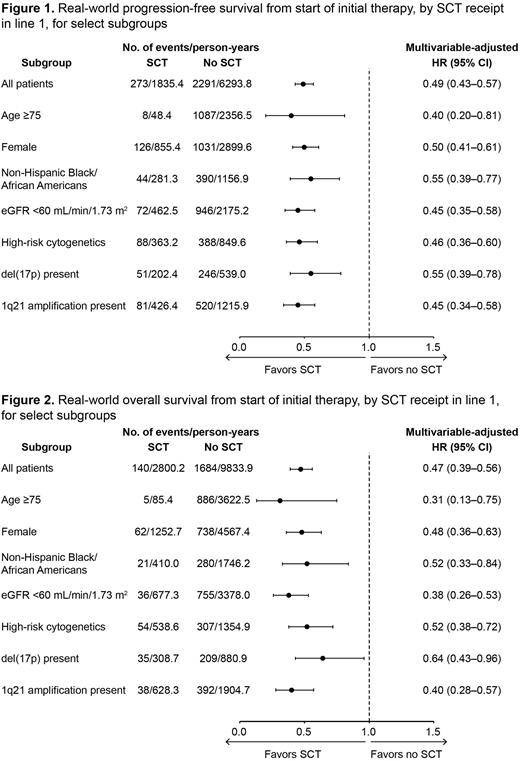
Results: At the time of data cutoff, 5996 pts had initiated front-line therapy; the median age was 70 years, 54.4% of pts were male, 55.8% were non-Hispanic White, 16.9% were non-Hispanic Black/African American, and 6.7% were Hispanic or Latinx. Overall, 1127 (18.8%) pts received SCT in line 1 and 4869 (81.2%) did not. Pts who received SCT were younger at diagnosis and start of first line of therapy than those who did not receive SCT (median 63 vs 72 years for both comparisons). Non-Hispanic Whites and Hispanic or Latinx pts were slightly more likely than non-Hispanic Black/African Americans to receive SCT in the first line (21.3% and 20.5% vs 16.5% of their respective populations). Receipt of SCT was more likely among pts with lower ISS disease stage at diagnosis and those treated in academic vs community settings; SCT was less likely among pts with renal impairment (estimated glomerular filtration rate <60 mL/min/1.73 m2) and poorer Eastern Cooperative Oncology Group performance status at start of front-line therapy. The most frequently prescribed initial therapy among pts both with and without SCT was a proteasome inhibitor (PI) + immunomodulatory drug (IMiD)-based regimen (in 72.2% and 49.0% of pts, respectively). PI-based (+/- steroids only) and monoclonal antibody-based (+/- steroids only) initial therapies were more common among pts who did not receive SCT in line 1 than those who did (15.8% vs 1.3% and 7.8% vs 3.8%, respectively). Compared to pts who did not receive SCT in line 1, those who received SCT in line 1 had longer rwPFS (adjusted hazard ratio [HR] 0.49; 95% confidence interval [CI] 0.43-0.57) and rwOS (adjusted HR 0.47; 95% CI 0.39-0.56). With both rwPFS (Figure 1) and rwOS (Figure 2), HRs were fairly consistent across all subgroups examined.
Conclusions: Among RW pts with NDMM from the Flatiron Health database, initial therapy with a PI + IMiD-based regimen was most common and roughly 1 in 5 pts received SCT in line 1. Receipt of SCT in line 1 was associated with longer rwPFS and rwOS, with the degree of benefit holding fairly consistent across all subgroups of pts examined, including those aged 75 years or older, women, non-Hispanic Black/African Americans, those with renal impairment, and those with high-risk cytogenetics [including del(17p) and 1q21 amplification].
Funding: Sanofi.
Disclosures
Richter:Secura Bio: Consultancy, Honoraria; Takeda: Consultancy; Oncopeptides: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Salinardi:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Rice:Sanofi: Current Employment, Other: may hold stock and/or stock options with Sanofi.
Author notes
Asterisk with author names denotes non-ASH members.