Abstract
Background: Approximately 50% of pediatric B-cell acute lymphoblastic leukemia (B-ALL) and non-Hodgkin lymphoma patients treated with CD19 chimeric antigen receptor (CAR)-T cells relapse. CD22 CAR-T cells are curative only rarely. New immunotherapeutic targets are urgently needed. Our laboratory recently identified CD72 as a target on B-cell malignancies and developed a fully synthetic nanobody against CD72 (NbD4), demonstrating that NbD4 CAR-T cells (nanoCARs) are potent in vitro and in vivo (Nix et al., Cancer Discovery (2021)). We previously described the development of a humanized version of this nanobody binder (H24) that had improved efficacy in preliminary studies (Nix et al., ASH 2021). As we move CD72 nanoCARs toward clinical translation, in this follow-up study we aimed to compare H24 vs. NbD4 by 1) tumor clearance kinetics; 2) T-cell immunophenotypes; 3) in vivo mechanism of relapse; and 4) binding affinity to CD72.
Methods: CD4/CD8 CAR-T cells were generated by lentiviral transduction. Single time point (24 hr) in vitro cytotoxicity was assessed across a range of effector:tumor (E:T) ratios. CAR-T elimination kinetics was determined by IncuCyte live cell imaging. CD45RA and CD62L flow cytometry was used to assess T-cell immunophenotypes. In vivo studies were performed by implanting 5e5 luciferase-labeled Jeko cells into NSG mice on Day -4, and administration of 3e6 CAR-T cells on Day 0. At sacrifice, flow cytometry was performed to evaluate CD72 expression on relapsed tumors. Relapsed Jeko was re-implanted into naïve NSG mice for 31 days. Binding affinity of recombinant NbD4 or H24 to biotinylated CD72 extracellular domain was determined by bio-layer interferometry.
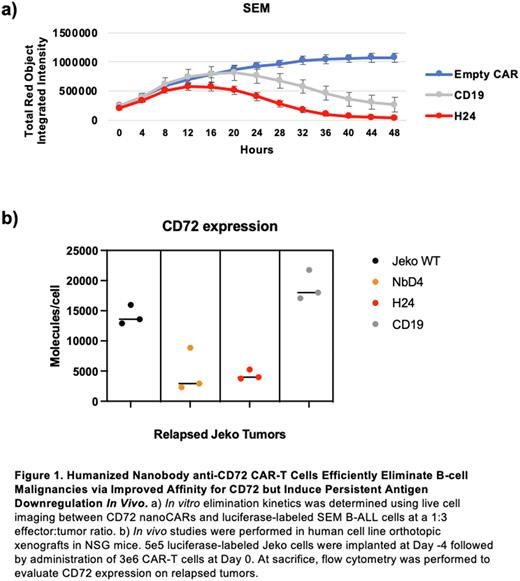
Results: In single time point assays, H24 nanoCARs eliminated SEM B-ALL cells with similar efficacy to CD19 CAR-Ts (not significant) and were more potent than NbD4 nanoCARs at a 1:10 E:T ratio (72% vs 44% cytotoxicity; p = 3e-4). Against a Jeko mantle cell lymphoma model, at a 1:1 E:T ratio, H24 nanoCARs were more potent than both CD19 CAR-Ts (96% vs 87%; p < 1e-4) and NbD4 nanoCARs (96% vs 70%; p < 1e-4). In live cell imaging assays, H24 nanoCARs eliminated SEM faster than CD19 CAR-Ts (Fig. 1a). After SEM co-culture, H24 nanoCARs secreted more IL-2 and IFNγ compared to NbD4 (IL-2 p < 1e-4; IFNγ p = 4e-4) and CD19 CAR-Ts (IL-2 p = 4e-4; IFNγ p = 3e-4). Versus SEM, both pre- and post-tumor proportions of naïve T cells (CD45RA+/CD62L+) were similar for H24, NbD4, and CD19 CAR-Ts. Bulk RNA-seq after exposure to SEM revealed that H24 nanoCARs upregulate genes relevant to T cell activation and effector T cell function, compared to CD19 CAR-Ts. In in vivo Jeko studies, we treated mice with NbD4 or H24 nanoCARs and extracted tumors after relapse. We found that both nanoCARs led to CD72 loss on tumors (Fig. 1b). Surprisingly, however, upon re-implantation into a new cohort of NSG mice, H24 relapse Jeko continued to maintain low CD72 expression, while re-implanted NbD4 relapse Jeko had restoration of CD72 in the absence of anti-CD72 immune pressure (H24 vs NbD4 CD72 expression p = 4e-2). Given more potent anti-tumor efficacy and more persistent CD72 suppression in murine models, we hypothesized that H24 has a higher binding affinity for CD72. Indeed, H24 demonstrated a dissociation constant (KD) versus CD72 of 33.0 ± 0.04 nM, while NbD4 was 50.4 ± 0.05 nM. These results support the notion that relatively low-affinity binders can still generate potent CAR-Ts, when compared to published values of KD <1 nM for the anti-CD19 scFv FMC63.
Conclusions: We have developed a new, humanized anti-CD72 cellular therapy known as H24 nanoCARs. Humanizing the framework regions, while keeping the complementary determining regions (CDRs) identical to NbD4, was intended to reduce immunogenicity. We were surprised that eight amino acid substitutions in the framework regions, with no changes to CDRs, improved antitumor efficacy and binding affinity for CD72. These improved characteristics motivate our planned clinical translation of H24-based nanoCARs. We also found that CD72 downregulation occurs after CD72 nanoCAR treatment in mice, and that increased potency of H24 correlates with longer-term CD72 suppression on tumors. These findings will inform expected outcomes in future clinical trials and motivate further studies to prevent CD72 downregulation after nanoCAR treatment.
Disclosures
Wiita:Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.