Abstract
Introduction DISC-0974 is a humanized monoclonal antibody against hemojuvelin (HJV) and is currently in Phase I clinical studies. HJV is a pathway-specific coreceptor for bone morphogenetic protein (BMP) signaling that regulates hepcidin expression and iron metabolism. Loss of function mutations of the HFE2 gene encoding HJV in humans cause profound reductions in hepcidin synthesis and severe iron overload. Consequently, it is hypothesized that pharmacological reduction of HJV activity will lead to reduction of hepcidin synthesis and increased iron availability. This hypothesis has been confirmed in studies of DISC-0974 in rodents and non-human primates and in healthy human volunteers. We have conducted studies to evaluate the effect of DISC-0974 in an animal model of CKD anemia.
Anemia is a common complication in patients with CKD and has been associated with multiple adverse outcomes in this population. In CKD hepcidin is increased because of both reduced renal clearance and increased synthesis. This increase is believed to be a central contributor to the development of anemia by reducing the availability of iron from systemic iron stores and by reducing dietary iron absorption. Supporting this hypothesis, treatment with hepcidin lowering drugs has been demonstrated to increase serum iron and to increase hemoglobin (Sheetz et al, Br J Clin Pharmacol. 2019;85:935-948). We hypothesize that by downregulating hepcidin, DISC-0974 will have beneficial effects in treating anemia in the CKD patients because it will make iron available for improved erythropoiesis and increased hemoglobin synthesis as well as reducing erythropoietin resistance.
Material and Methods The effect of DISC-0974 on improving anemia was evaluated in an adenine-induced rat model of CKD. In this study, renal impairment was induced by giving Sprague Dawley rats a diet containing 0.75% adenine for 3 weeks to induce kidney injury and then switched to a control diet for the remainder of the study (i.e., 3 more weeks). DISC-0974 (20 mg/kg) or vehicle (n-5/group) was administered intravenously once per week starting on Day 7 and continuing to Day 35. An additional group of rats (n=5/group) was fed with the control diet for the entire study and dosed intravenously with vehicle. Rats were euthanized on Day 42. Blood was collected on Days 0, 7, 21, 35, and 42 for evaluation of hematology parameters, as well as serum iron and hepcidin. Livers were also collected at study end for evaluation of hepcidin gene (HAMP) mRNA expression level.
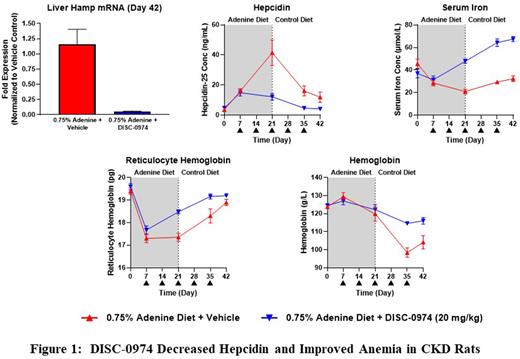
Results Consistent with a previous report (Sun et al, Nephrol Dial Transplant. 2013;28:1733-1743), the 0.75% adenine diet induced kidney dysfunction, as evidenced by marked increase in both serum creatinine and blood urea nitrogen. Rats on adenine diet developed reduced serum iron, reduced red cell production, and lowered hemoglobin with a hypochromic microcytic profile reflecting reduced iron availability.
CKD rats appeared to tolerate DISC-0974 treatment well, as DISC-0974 @ 20mg/kg did not affect body weight.
Consistent with the mechanism of action of blocking the formation of the BMP/BMPR/HJV complex, DISC-0974 reduced HAMP gene expression in the liver as measured at the end the study, which led to reduced serum hepcidin and increased serum iron concentration. Cellular hemoglobinization measured as reticulocyte Hemoglobin was increased. The maximum improvement in hemoglobin by DISC-0974 compared to the vehicle group was 17g/L in the rat CKD model.
Conclusions We conclude that treatment with DISC-0974 at a dose that reduces hepcidin gene expression is able to increase iron availability and substantially prevents the reduction in hemoglobin that is seen in animals that develop adenine-induced renal impairment. This study supports the development of DISC-0974 for the treatment of patients with CKD anemia.
Disclosures
Wu:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Wang:DISC Medicine: Current Employment, Current holder of stock options in a privately-held company. MacDonald:Disc Medicine: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.