Abstract
Heparin-induced thrombocytopenia (HIT) is a severe adverse event that is caused by the interaction of platelets with ultra-large platelet factor 4 (PF4)/heparin IgG-immune complexes. Previous studies have shown that HIT Abs harbour the potential to interact with PLT Fc-gamma-RIIA leading to PLT activation and interplay with different actors of the innate and adaptive immune system. Despite this mechanistic insights, the role of different HIT Ab-induced PLT subpopulations that might contribute to an increased pro-thrombotic potential and severe patient outcome, remains poorly understood.
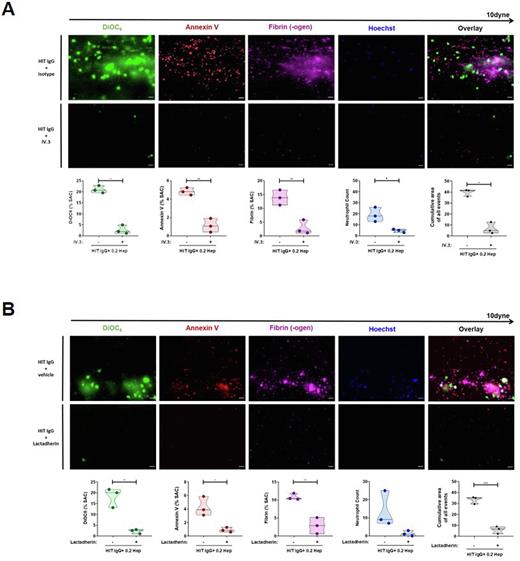
In this study, we hypothesized that PF4/heparin HIT Abs induce a procoagulant PLT response that contribute to an increased pro-thrombotic environment. Using flow cytometry, we found that HIT patient Abs have the capability to induce a procoagulant (P-Selectin/phosphatidylserine double positive) PLT phenotype (p=0.0039) with increased thrombin generation potential (p<0.0001). Most importantly, the perfusion of whole blood samples that were spiked with HIT Ab-induced procoagulant PLTs resulted in significant increase of procoagulant (Annexin-V positive) PLT and fibrin deposition. Interestingly, Fc-gamma-RIIA inhibition via monoclonal antibody IV.3 resulted in a nearly complete abrogation of HIT Ab-induced thrombus formation, indicating that PLT Fc-gamma-RIIA signalling mechanisms are pivotal for Ab-induced pro-thrombotic PLT potential (p=0.0108, Figure 1A). Moreover, also the prostacyclin analogue Iloprost prevented procoagulant PLTs and increased thrombus formation despite the presence of HIT Abs and low dose (0.2 IUs/mL) heparin (p=0.0029). A finding that might be most probably due to increased intracellular concentrations of the second messenger cAMP due to increased PKA activity.
To deeper investigate how increased CD62p expression and high PS externalization on the procoagulant PLT surface contribute to the prothrombotic potential of HIT Ab-induced procoagulant PLTs, we performed specific inhibition experiments. Although a marked reduction of neutrophil granulocyte deposition was observed, blocking of PLT CD62p did not reduced HIT Ab-induced increased thrombin generation or thrombus formation. In contrast, procoagulant PLTs that were treated with lactadherin, a PS binding and blocking protein resulted in a significant reduction of thrombin generation (p=0.0040) and thrombus formation compared to the vehicle control (p=0.0005, Figure 1B).
Findings of our study indicate that HIT patient Abs harbour the ability to induce procoagulant PLTs via PLT FcγRIIA mediated signalling pathways. The observation that Iloprost reduced the formation of procoagulant PLTs and most importantly inhibits thrombus formation indicates a potential therapeutic use. Most importantly, based on our mechanistic studies we conclude that increased PS on the surface of procoagulant PLTs might be the causing factor for increased pro-thrombotic potential observed in HIT.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.