Abstract
Background: Vitamin K clotting factor deficiency (VKCFD) types 1 and 2 are very rare autosomal recessive disorders associated with reduced clotting factors activities for all vitamin K dependent clotting factors. Less than 30 cases are reported with defects in GGCX (gamma-glutamyl carboxylase) leading to VKCFD Type 1 (VKCFD1) or VKORC1 (vitamin K epoxide reductase complex subunit 1) leading to VKCFD Type 2 (VKCFD2). In addition to clotting factor deficiency, GGCX mutations can be associated with skeletal abnormalities such as bone hypoplasia, conductive hearing loss, mental retardation, and skin hyperlaxity. Here, we report an Arabic family with VKCFD1 harboring an unreported, new pathogenic variant in the GGCX gene. The proband presented at 17 months of age with severe bruising following trauma and work up demonstrated deficiencies of all Vitamin K dependent (VKD) coagulation proteins. Another sibling born 8 years later was also diagnosed with the same condition with abnormal coagulation parameters at birth. Additionally, the non-hemorrhagic manifestations diagnosed in both siblings were mid-facial hypoplasia with flat nasal bridge, dental abnormalities, and shortened distal phalanges representing a keutel syndrome-like phenotype. Both patients are currently managed with oral vitamin K supplementation with resolution of bleeding symptoms. Here we present the in-vitro assessment of the mutations effects to vitamin K supplementation and potential clinical implications.
Methods: The proband, affected brother and mother, all underwent genetic evaluation at the University Clinic Bonn in Germany. DNA was isolated from the patient's blood and Sanger Sequencing was performed for the genes VKORC1 and GGCX. The identified new pathogenic variant in GGCX was further characterized by the in-vitro assay published by Ghosh et al. in 2021.
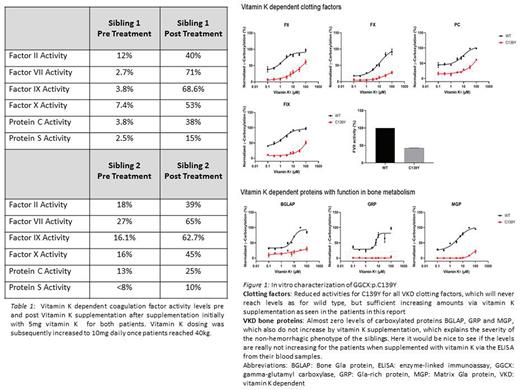
Results: The brothers were found to be homozygous for c.[416G>A];[416G>A] p.(Cys139Tyr), a genetic variant in exon 4 of the GGCX gene. Mutations in VKORC1 gene leading to reduced activity for the VKD clotting factors were excluded confirming the diagnosis of VKCFD Type 1 (VKCFD1). The mother was found to be heterozygous for the same GGCX variant. The affected siblings and the mother were further genotyped for the promoter single nucleotide polymorphism in VKORC1 gene NG_011564.1:g.3588G>A (VKORC1:c.-1639 G>A) since this might correlate with the severity of the phenotypes in patients with skeletal manifestations. The brothers and mother were found to harbor VKORC1:c.-1639 GA, which has been associated with a 33% reduced VKORC1 expression. Treatment with 5mg of vitamin K resulted in increased activities of the VKD clotting factors (Table 1). However, clotting factor activities did not normalized after vitamin K supplementation.
The in vitro characterization of the new identified pathogenic variant GGCX:p.C139Y (Figure 1) showed reduced activities for all VKD clotting factors, which did not reach wild type levels despite increasing concentrations of vitamin K. The levels of Matrix Gla protein (MGP), Bone Gla protein (BGLAP), and Gla-rich protein (GRP) showed almost no response to increasing amount of vitamin K.
Discussion: Here, we report a new pathogenic variant in exon 4 of GGCX c.[416G>A];[416G>A] p.(Cys139Tyr) leading to VKCFD1 with reduced clotting factor activities and non-hemorrhagic manifestations. The pre and post vitamin K supplementation factor levels in the patients correlate with the in-vitro data and demonstrate failure of the clotting factor levels to normalize despite vitamin K supplementation. The in vitro data for the non-hematological VKD proteins indicate that the patients might develop a pseudoxanthoma elasticum (PXE)-like phenotype resulting into hyperlaxity of skin due to the almost zero levels of carboxylated GRP. The skeletal/bone phenotypes can be explained by the almost zero values for carboxylated MGP and GRP. To assess this in-vivo, levels of human MGP and BGLAP are currently being analyzed in the patients and mothers blood samples. These patients highlight the nuances associated with the diagnosis and management of patients with VKCFD. The antenatal management and timing of Vitamin K supplementation to prevent intrauterine bleeding is also unknown, and there may be benefit to starting early to prevent the bone and skin manifestations. Further research is warranted in rare bleeding disorders such as VKCFD.
Disclosures
Pezeshkpoor:Octapharma: Other: Consultant, Research Funding, Speakers Bureau; Biotest: Research Funding; Novo Nordisk: Other: Consultant; Bayer: Research Funding. Oldenburg:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; Grifols: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Biogen Idec: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; University Clinic Bonn: Current Employment; Freeline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Biomarin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; Biotest: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; Chugai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; CSL Behring: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Sparks: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau. Chitlur:BPL Inc: Honoraria; Emerging Therapeutics Inc: Honoraria; Sanofi/Genzyme Corp: Honoraria; Genentech Pharmaceuticals: Honoraria, Research Funding; Agios Pharmaceuticals: Honoraria, Research Funding; NovoNordisk: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.