Abstract
Introduction: Platelet transfusion refractoriness due to HLA alloimmunization occurs in up to 20% of patients with hematologic malignancies and can present significant diagnostic and management challenges. In addition, institutional practices may vary depending on access to testing platforms and donor pools. Typically, once alloimmune refractoriness to platelet transfusions is confirmed clinically, a percentage calculated panel reactive antibody (cPRA) is determined based on HLA antibody testing. Subsequently, HLA compatible platelet transfusions are initiated and continued for as long as is clinically indicated. Repeat HLA antibody testing is not routinely performed due to relatively high costs. At our institution, we recommend serial HLA antibody measurement (and cPRA monitoring) every 1-2 months in all patients who require HLA compatible products as a way to proactively narrow or expand the donor pool. In order to formally assess the clinical utility of this practice, we studied cPRA trends in patients with hematologic malignancies over a 1-year period following initial HLA antibody testing.
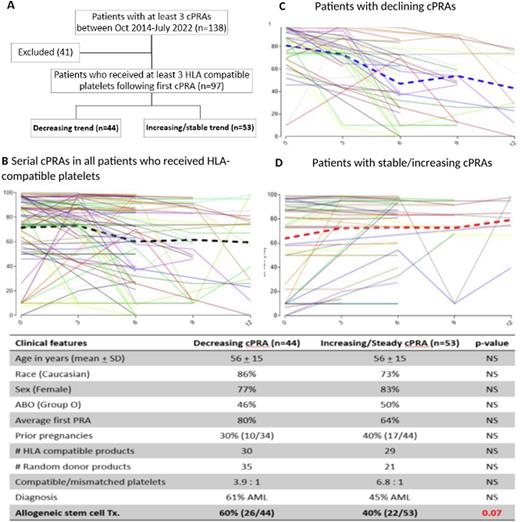
Methods: At the Fred Hutchinson Cancer Center, from October 2014 to July 2022, we retrospectively reviewed electronic health records of patients with hematologic malignancies who had at least 3 serial HLA antibody identification tests over the course of a year due to platelet refractoriness. HLA antibodies were identified using a Luminex single antigen bead assay and reported as positive when the Mean Fluorescence Intensity (MFI) were over 2,500. CPRA was calculated with these HLA specificities. Of these individuals who were HLA-alloimmunized, we further identified ones who required 3 or more HLA-compatible platelet transfusions during the same year (Figure 1A).
Results: Ninety-seven patients were identified as being HLA-allo immunized and platelet transfusion refractory in the study. Mean age of the cohort was 57 years (SD +15). A majority of the patients were Caucasian (81%; n=79/97) and 80% (n=78/97) were female. The 3 most common diagnoses were acute myelogenous leukemia (AML) (53%), myelodysplastic syndrome (14%), and aplastic anemia (7%). Of all patients, 50% (48/97) had undergone one or more allogeneic hematopoietic stem cell transplantations (HSCT). A third of the women (26/78) had a previous history of pregnancy. Mean number of HLA compatible and pooled/random donor platelets transfused during the study period were 30 (+ 29) and 28 (+51) units, respectively.
Overall, the average cPRAs declined significantly from 72% to 60% by 6 months (p=0.01) (Figure 1B) on serial HLA-antibody testing. Declining and increasing/stable trends in cPRA for individual patients are shown in Figures 1C and 1D, respectively. When clinical data were stratified by the 2 groups (those with declining and increasing/stable serial cPRAs) (Table), there was a marginally higher proportion of subjects who had undergone allogeneic HSCT in the "declining cPRA” group compared to the group which demonstrated "stable or increasing cPRA” trends. No other differences were identifiable.
Conclusion: Periodic assessment of HLA antibodies demonstrated a significant decline in cPRA in HLA alloimmunized patients with hematologic malignancies requiring chronic HLA-compatible platelet transfusions. This was more prominent among patients who had undergone allogeneic HSCT. Our data are consistent with a previous subgroup analysis (Slichter et al. Transf. Med. Rev. 2011) which showed that more than half of the HLA-alloimmunized patients will lose their antibodies before 12 months. Moreover, HLA antibody testing methods used in our patient cohort are more relevant to the current context. In all HLA-alloimmunized platelet transfusion refractory patients with hematologic malignancies, proactive periodic HLA antibody testing may help broaden the limited donor pool and rechallenge patients with platelet products containing previously incompatible HLA antigens. Further studies are ongoing to determine whether specific antibody types/classes demonstrate a predilection to wane or intensify over time in this retrospective cohort.
Disclosures
Hess:stockholder and inventor in MedCura, Inc, consultant to Hemerus, LLC: Consultancy, Current equity holder in private company. Gernsheimer:Amgen Corporation: Consultancy; Cellphire Corporation: Consultancy; Sanofi: Consultancy; Palisade Bio: Consultancy; Dova Corporation: Consultancy; Principia: Consultancy; NHLBI: Other: Grant Funding. Panch:Consultancy for SOBI-SANOFI Pharmaceuticals: Other: Consultancy for SOBI-SANOFI Pharmaceuticals.
Author notes
Asterisk with author names denotes non-ASH members.