Abstract
Acidosis and hypoxia are common hallmarks of cancer associated with poor therapy response and dismal survival. Hypoxia can be due to high demand on oxygen, or due to reduced oxygen supply consequent to pharmacological inhibition of oxidative phosphorylation (OxPhos), causing switch to glycolysis and intracellular acidosis. Both acidosis and hypoxia are known to activate AMPK, stabilize HIF1α, that might orchestrate metabolic switch to alternative sources of energy such as lipophagy, an autophagic degradation of intracellular lipid droplets.
We have previously shown that co-inhibition of mitochondrial Complex I and pyruvate-lactate transporter 1 (MCT1) in T-ALL cells is synergistic both in vitro and in vivo (Baran N, et al. ASH 2021). Here we dissect the mechanism of OxPhos/MCT1 blockade (OxPhos/MCT1-i), showing lipophagy as a mechanism of resistance to dual inhibition and provide therapeutic strategy to eradicate the residual disease.
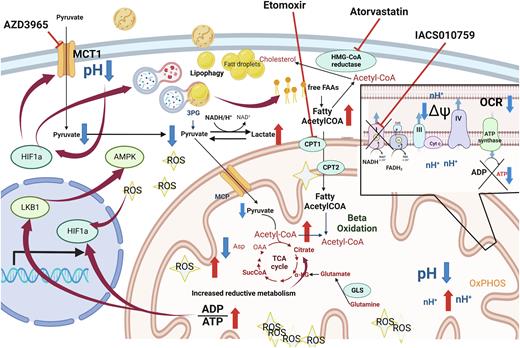
In T-ALL cell line models, Complex I/MCT1 blockade led to a significant inhibition of viability and decreased cell proliferation. These effects were accompanied by significant accumulation of reactive oxygen species (ROS) and mitochondrial superoxide, leading to DNA damage and apoptosis (shown by γ-H2AX and cleaved PARP). High level of ROS remained elevated despite co-treatment with ROS-scavengers: ascorbate, α-ketoglutarate, GSH or NAC. To elucidate the mechanisms of cell death, cells were co-treated with inhibitors of autophagy (3-methyladenine, Bafilomycin A, Chloroquine, Wortmannin), apoptosis (QVD-OPH, ZVAD-fmk) and ferroptosis (Ferrostatin1). While all inhibitors improved cell viability, suggesting a mixed contribution of apoptosis, autophagy and ferroptosis, none rescued elevated ROS caused by OxPhos/MCT1-i. Further impairment of mitochondria and Oxphos was evidenced by loss of mitochondrial membrane potential, mitochondria swelling, and reduction of mitochondria number, associated with markers of mitochondria fusion (elevated OPA1, MFN1 and DRP1). Significant reduction of ATP was confirmed by Seahorse and mass spectrometry (MS), indicating TCA cycle blockade and depletion of glutamine, cystathionine and glutathione, likely in response to ROS. ROS and ATP depletion are known to stabilize the hypoxia inducible factor 1 (HIF1α) that controls triglyceride accumulation. Results of GSEA and Western blotting confirmed that dual inhibition induced energetic crisis, activated LKB-AMPK pathway, inhibited mTOR pathway, upregulated fatty acids metabolism, which together with ROS accumulation caused HIF1α stabilization and switch to glycolysis (elevated ECAR by Seahorse and lactate by MS). Glycolysis however, impaired by MCT1 blockade, led to intracellular acidification, reflected by pH Rodo staining. This was associated with significant increase of a key component in lipid turnover acetyl-CoA, and by increased intensity of BODIPY dye, accompanied by increase in level of cellular lysosomes detected by Lysotracker dye, suggesting a possible shift of metabolic dependency toward lipophagy as a remaining source of energy. These results indicate that OxPhos/MCT1-i triggers storage of reduced lipids which subsequently undergo lysosomal degradation during lipophagy, constituting an alternative to OxPhos source of energy.
To test if inhibition of lipid metabolism might abrogate the last energetic supply and eradicate residual leukemic cells, T-ALL cells were co-treated with OxPhos/MCT1-i together with Atorvastatin, Etomoxir and Valproate. Concomitant administration of Atorvastatin as well as other inhibitors with both OxPhos/MCT-i significantly reduced cell viability and induced apoptosis, supporting the key role of lipid metabolism in the resistance of T-ALL to dual inhibition.
In summary, these results demonstrate that OxPhos/MCT1 inhibition forces switch to lipophagy, sensitizing cells to inhibitors of lipid metabolism, uncovering metabolic checkpoints that can ultimately translate into successful therapies in T-ALL and OxPhos-dependent malignancies.
Disclosures
Skwarska:NOVARTIS: Other: PATENT. Konopleva:Stemline Therapeutics, F. Hoffman La-Roche; Janssen: Membership on an entity's Board of Directors or advisory committees; Reata Pharmaceuticals, Novartis and Eli Lilly: Patents & Royalties; Forty-Seven; F. Hoffman LaRoche: Honoraria; AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven: Research Funding; Stocks, Reata Pharmaceuticals: Current equity holder in publicly-traded company; AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.