Abstract
Nerve-driven chronic adrenergic stress can suppress anti-tumor immunity. However, the mechanistic details of how this occurs are still poorly understood. Catecholamines (epinephrine, norepinephrine) released by the autonomic nervous system (ANS) can activate adrenergic receptors (AR) present on nearly every cell type, including myeloid derived suppressor cells (MDSCs). MDSCs are important regulatory cells that hinder anti-tumor immune responses in hematological malignancies by inhibiting numerous cell types, including effector NK cells, CD8+ T cells, CD4+ T cells, and processes, such as blocking cytokine secretion. Because first line treatment for most hematologic malignancies includes chemotherapy, and MDSCs are known to be detrimental to its efficacy, understanding their role, including through chronic stress pathways, is relevant.
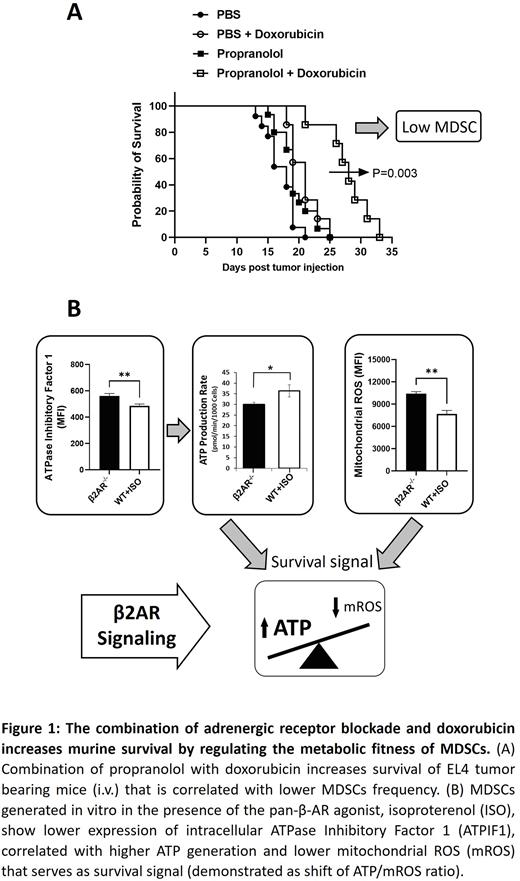
Recently, using preclinical models of solid tumors, we have shown β2 adrenergic receptor (β2-AR) signaling, activated by chronic stress, increases the accumulation of MDSCs in the tumor microenvironment (TME). However, the role of β2-AR in MDSC survival and accumulation in hematological malignancies after chemotherapy, and the precise mechanisms by which β2-AR increases MDSC accumulation, remain unresolved. We have discovered β2-AR signaling drives MDSC survival in tumor bearing mice treated with chemotherapy. We have also shown the combination of propranolol, a non-selective β-AR blocker, with doxorubicin prolongs survival in mice bearing EL4 and C1498 tumors. Examination of immune cell compartments demonstrated a significant reduction of both M-MDSC and PMN-MDSC abundance in mice receiving the combination therapy, indicating the key effect of stress induced β2-AR signaling on MDSC accumulation. Further, we implanted EL4 tumors into β2-AR-/- and β2ARfl/fl LysmCre mice and confirmed doxorubicin increases survival in both β2-AR-/- and β2ARfl/fl LysmCre mice compared to WT control mice, suggesting β2-AR signaling in MDSCs is the main receptor driving the effects of propranolol in combination therapy.
Using both in vitro and in vivo systems, we showed β2-AR signaling increased MDSC survival by the induction of BCL-2 expression and the suppression of caspase-3 and caspase-8. Seahorse metabolic studies further showed β2-AR signaling induced a metabolic shift to increased mitochondrial respiration (OCR) and ATP production in MDSCs.
We then applied single cell RNA sequencing (scRNAseq) pathway analysis and multiplexed D7-Glc plus 13C5-Gln tracing in Stable Isotope-Resolved Metabolomics (SIRM). We found β2-AR signaling induces a metabolic shift toward higher ATP generation and decreased mitochondrial reactive oxygen species (mROS) that serves as a survival signal for MDSCs. We also found β2-AR signaling suppressed the expression of ATPase Inhibitory Factor 1 (ATPIF1) in MDSCs, which is responsible for increased ATP generation. These results show β2-AR signaling induces MDSC survival through metabolic reprogramming in mitochondria by enhancing ATP generation and modulating key survival signals to increase the ratio of ATP/mROS.
Altogether, these studies reveal β2-AR signaling plays an important role in mitochondrial metabolic fitness in MDSCs, priming them to survive chemotherapy-induced cytotoxicity. The combination of the stress blocker propranolol, with chemotherapy regimens, could potentially improve the current therapeutic regimens used for hematological malignancies.
Disclosures
McCarthy:Novartis: Consultancy, Honoraria; Fate Therapeutics: Consultancy, Honoraria; Magenta Therapeutics: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria; Takeda Pharmaceuticals America, Inc.: Consultancy, Honoraria; Partner Therapeutics, Inc.: Consultancy, Honoraria; Bluebird Bio: Consultancy, Honoraria; Juno: Consultancy, Honoraria; Axios: Consultancy, Honoraria; Starton Therapeutics: Consultancy, Honoraria; Sanofi: Consultancy; Abbvie: Consultancy, Honoraria; Karyopharm Therapeutics Inc.: Consultancy, Honoraria; Janssen Global Services, LLC: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.