Abstract
Background: Approximately half of adults with acute myeloid leukemia (AML) are unable to receive standard intensive chemotherapy (IC) due to older age or comorbidities (Griffiths et al. Leuk Res. 2020;91:106339). Although age is prognostic, several factors define patient fitness, such as comorbidities and cytogenetic risk. A better determination is needed to assess patient fitness for AML treatment regimens as there is no currently defined standard. The AML composite model (AML-CM) is a validated system used to determine fitness for transplant and potentially other intensive regimens. The randomized double-blind phase 3 VIALE-A trial demonstrated that in older adults who were unfit for IC based on modified Ferrara criteria, venetoclax (VEN), an oral BCL-2 inhibitor, combined with azacitidine (AZA) increased median overall survival (mOS) to 14.7 mo compared with 9.6 mo with placebo (PBO)+AZA. However, the Ferrara criteria contain limited patient characteristics to ascertain fitness for IC. Therefore, we aimed to assess the benefit of VEN+AZA vs PBO+AZA in patients with differential fitness and disease biology across the spectrum of AML-CM score.
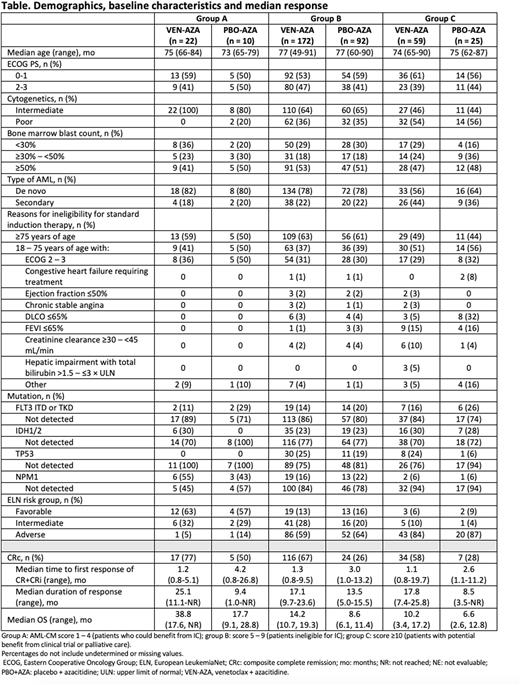
Methods: VIALE-A (NCT02993523) evaluated the efficacy of VEN+AZA compared to PBO+AZA in patients with treatment-naive AML who were ineligible for IC due to age or comorbidities. Patients (N = 431) were randomized 2:1 to receive VEN+AZA or PBO+AZA, respectively. AML-CM score was determined retrospectively based on age, comorbidities, and cytogenetic risks (Sorror et al. JAMA Onc. 2017;3:1675). Patients in each treatment arm were stratified into 3 risk groups by AML-CM scores: group A, score 1 - 4 (patients who could potentially benefit from IC); group B, score 5 - 9 (patients with decreased benefit from IC); and group C, score ≥10 (patients who could potentially benefit from a clinical trial or palliative care).
Results: Of 431 patients enrolled to VIALE-A, 380 were categorized by AML-CM score and 51 could not be categorized due to missing data. Missing data included ELN risk, obesity, hepatic comorbidities, and hypoalbuminemia. Some patients with missing data were included in the analysis group if that data would not have impacted AML-CM group assignment. The proportion of patients in AML-CM groups A, B, and C, respectively, was 22 (9%), 172 (68%), and 59 (23%) in the VEN-AZA arm and 10 (8%), 92 (72%), and 25 (20%) in the PBO-AZA arm. Patients with higher AML-CM scores generally had poor prognostic disease characteristics, adverse ELN risk, decreased pulmonary function, and other comorbidities (Table). Composite complete remission (CRc [CR + CRi]) rates (groups A/B/C) were 17 (77%)/116 (67%)/34 (58%) with VEN-AZA, and 5 (50%)/24 (26%)/7 (28%) with PBO-AZA (Table). The median time to first CRc response in the VEN-AZA arm was 1.2 mo for group A, 1.3 mo for group B, and 1.1 mo for group C. In the PBO-AZA arm, the median time to first CRc was 4.2 mo, 3.0 mo, and 2.6 mo for groups A, B, and C, respectively (Table). The median duration of CRc across groups A, B, and C was 25.1 mo, 17.1 mo, 17.8 mo, respectively, in the VEN-AZA arm and 9.4 mo, 13.5 mo, and 8.5 mo in the PBO+AZA arm (Table). The mOS was 38.8 mo, 14.2 mo, and 10.2 mo in groups A, B, and C, respectively, in the VEN+AZA arm, and 17.7 mo, 8.6 mo, and 6.6 mo, respectively, in the PBO+AZA arm (Table).
Early deaths within 30 days in groups A, B, and C, respectively, were 0 (0%), 9 (5%), and 8 (14%) in the VEN-AZA arm, and 0 (0%), 2 (2%), and 3 (12%) in the PBO+AZA arm. Of 22 deaths across both treatment arms in all AML-CM groups, there was 1 death within 30 days (VEN+AZA, group B) that was due to disease progression, and 21 deaths within 30 days that were due to adverse events.
Conclusion: Based on the AML-CM score, most patients enrolled in VIALE-A were considered to have decreased benefit from IC, and nearly a quarter of patients evaluated were determined to be the frailest. Under this scoring system, patients in VIALE-A treated with VEN+AZA had higher CRc rates and prolonged duration of CRc and OS compared with those treated with PBO+AZA across all 3 AML-CM groupings. Notably, patients who scored >10 on the AML-CM scale, for whom the recommended treatment is enrollment in clinical trials and/or palliative care, had improved outcomes with VEN+AZA compared with PBO+AZA.
Disclosures
Venditti:Jazz Pharmaceuticals: Honoraria, Research Funding; Pfizer: Honoraria, Speakers Bureau; astrazeneca: Honoraria; Astellas: Membership on an entity's Board of Directors or advisory committees; abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Medac: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen & Cylag: Honoraria; Servier: Membership on an entity's Board of Directors or advisory committees. Fenaux:Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria. Jonas:GlycoMimetics: Consultancy, Other: protocol steering committee , Research Funding; Gilead: Consultancy, Other: data monitoring committee , Research Funding; AbbVie: Consultancy, Other: Travel Reimbursement, Research Funding; 47: Research Funding; Genentech: Consultancy, Research Funding; Jazz: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Servier: Consultancy; Takeda: Consultancy; Tolero: Consultancy; Treadwell: Consultancy; Accelerated Medical Diagnostics: Research Funding; Amgen: Research Funding; AROG: Research Funding; BMS: Consultancy, Research Funding; Celgene: Research Funding; Daiichi Sankyo: Research Funding; F. Hoffmann-La Roche: Research Funding; Forma: Research Funding; Roche: Research Funding; Hanmi: Research Funding; Immune-Onc: Research Funding; Incyte: Research Funding; Loxo Oncology: Research Funding; LP Therapeutics: Research Funding; Pharmacyclics: Research Funding; Sigma Tau: Research Funding. Vrhovac:Abbvie, Astellas, Pfizer, Pharmas: Consultancy; Abbvie, Astellas, MSD, Novartis, Pfizer, Pharmas, Servier, Teva: Speakers Bureau. Montesinos:Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Research Funding; Astellas: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Menarini/Stemline: Consultancy, Research Funding; Otsuka: Consultancy; Kura Oncology: Consultancy; Incyte: Consultancy; Ryvu: Consultancy; Nerviano: Consultancy; Beigene: Consultancy. Garcia:AbbVie, Genentech, AstraZeneca, Prelude and Pfizer: Other: Clinical trial support (institutional) , Research Funding; AbbVie, Astellas, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board ; AbbVie: Research Funding. Rizzieri:AbbVie: Honoraria, Speakers Bureau. Thirman:AbbVie, AstraZeneca, Celgene,Janssen, Pharmacyclics, Roche/Genentech: Consultancy; AbbVie,Gilead Sciences,Janssen,Merck,Pharmacyclics, Syndax, TG Therapeutics, Tolero Pharmaceuticals.: Consultancy, Research Funding. Montez:Roche: Current equity holder in publicly-traded company; Genentech/Roche: Current Employment, Current equity holder in publicly-traded company. Liu:AbbVie: Current Employment, Current equity holder in publicly-traded company. Katsetos:AbbVie: Current Employment, Current equity holder in publicly-traded company. Potluri:AbbVie: Current Employment, Current equity holder in publicly-traded company. Miller:AbbVie: Current Employment, Current equity holder in publicly-traded company. Pullarkat:Amgen, Dova, and Novartis: Consultancy, Other: Advisory Board Member; AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.