Abstract
Background: Dexamethasone is an anti-inflammatory steroid used with chemotherapy to prevent nausea/vomiting. Dexamethasone reduces chemo-resistance through inhibition of cytokine production and proliferation via nuclear factor kappa B (NF-kB) dependency. NF-kB is a pro-inflammatory transcription factor. When inactivated, NF-kB complexes are sequestered by inhibitors of kappa-B (I-kB) in the cytoplasm. Upon activation, NF-kB dimers translocate to the nucleus, activating kB responsive DNA. In AML, it has been found that NF-kB is highly activated, suggesting it is an integral part of AML biology. Approximately, 40% of patients diagnosed with AML have demonstrated increased activity of NF-kB and enhanced chemoresistance. In this study, we investigate the effect of dexamethasone given as adjuvant antiemetic prophylaxis on clinical AML outcome.
Methods: A single-center, retrospective, descriptive study, from May 2015 to September 2021, was conducted in AML adult cohort admitted for induction therapy at Baylor St. Luke's Medical Center. Dexamethasone exposure was defined as a dose at least 8 mg daily for a total of 5-7 days during induction therapy and consolidation. Primary endpoint was clinical efficacy including event free survival (EFS) and overall survival (OS). Secondary endpoints were complete remission (CR) in patients receiving dexamethasone vs. not. Statistical analysis using SAS software included descriptive and inferential statistics.
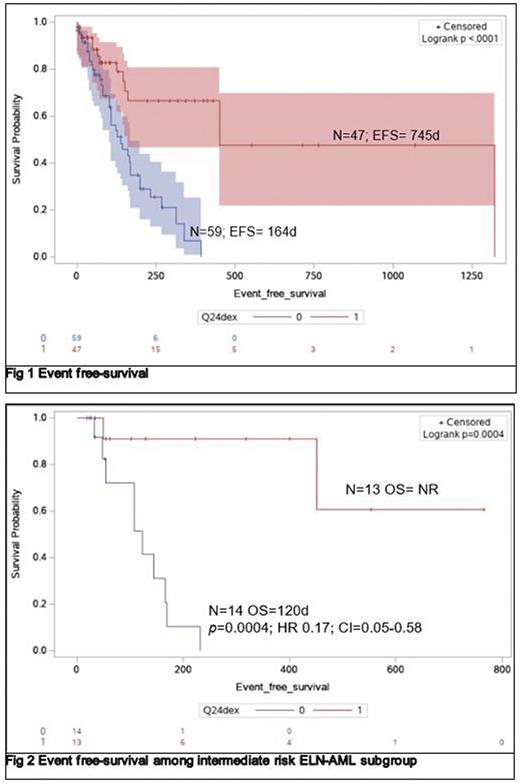
Results: 106 patients were evaluated. 47 (44.3%) were exposed to dexamethasone. Median age was 61 years (21-88) vs 55 years (26-82), p=0.03. ELN-2017 distribution among dexamethasone exposed vs. those who were not was in favorable 32% vs 15%, intermediate 32% vs 26 % and unfavorable 37% vs 58%. EFS was 745 days (d) vs. 164 d, in patients receiving dexamethasone vs. not, p=0.0001, HR=0.31, CI=0.16-0.54 (Fig 1). Subgroup analysis by ELN-2017 classification demonstrated that superior EFS was driven by intermediate risk, p=0.0004, HR=01, CI= 0.03-0.3 (Fig 2). In de novo AML, EFS was not reached (NR) vs 162d in AML patients dexamethasone exposed vs. not exposed (p=0.0001, HR=0.12, CI=0.05-0.29). Similarly, for secondary AML, EFS was 1,322 d vs 199 d, in dexamethasone exposed vs not exposed, p=0.05, HR=0.37, CI=0.14-0.92. CR/CRi was 60 % vs 40.2% for patients dexamethasone vs. not, p=0.2. With EFS, as outcome of interest, cox regression model was constructed accounting for age, ELN-2017 classification, and dexamethasone exposure demonstrating that favorable ELN-2017 (p=0.004; HR=0.17; CI=0.05-0.58) and exposure to dexamethasone (p=0.001; HR=3.7, CI=1.6-8.2) interacted to induce superior EFS.
Conclusions: Dexamethasone exposure is associated with significant lower relapse risk in adult diagnosed with AML receiving induction. Our study is "proof of concept” for dexamethasone activity against AML. Albeit activity seems restricted to intermediate ENL-2017 subgroup, mitigation of relapse risk seems wide among de novo and secondary AML. Clinical studies are needed to reconfirm our findings and to uncover the inflammatory pathways targeted by dexamethasone leading to anti-leukemia effect.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.