Abstract
Introduction: JAK2V617F is the most frequent mutation in BCR-ABL-negative myeloproliferative neoplasms (MPNs) and is an important determinant of MPN phenotype. Although several studies revealed clonal evolution of MPN at the hematopoietic stem cell (HSC) level, few studies have focused on phenotypic heterogeneity in downstream differentiation. The aim of this study is to find possible therapeutic targets by molecular analysis of JAK2V617F-positive MPN at HSC and megakaryocyte-erythroid progenitor (MEP) levels.
Methods: Transcriptomic analysis was performed on isolated bone marrow (BM) HSCs and MEPs from 12 JAK2V617F+ ET, 9 JAK2V617F+ PV, and 8 HD as controls. Differentially modulated signaling pathways during hematopoietic differentiation from HSCs to MEPs were identified. To model the role of Wnt/β-catenin signaling in MPN clonal evolution, CD34+ cells from MPN patients were cultured in vitro with or without the presence of Wnt/β-catenin inhibitor (XAV939). JAK2V617F+ transgenic mice (C57BL/6, 8-10 weeks) were injected with XAV939 intraperitoneally every day for 6 weeks to verify the role of inhibiting Wnt/β-catenin signaling in thrombocytosis remission in vivo.
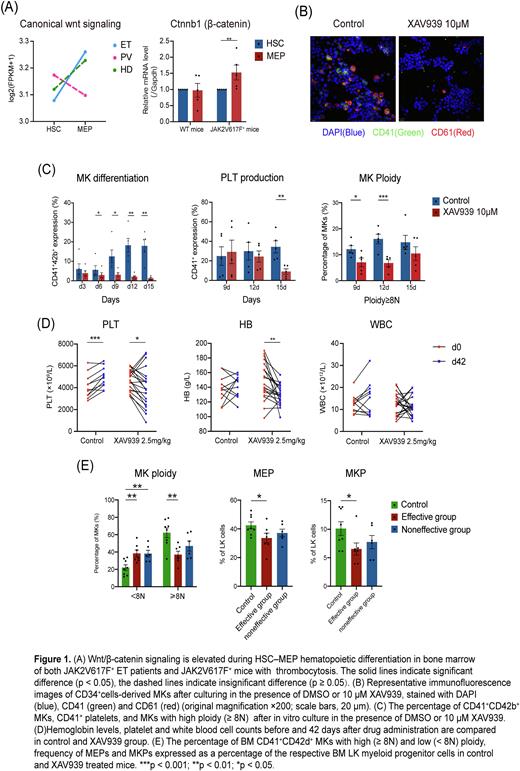
Results: GSEA analysis identified signaling pathways that changed during HSC differentiation to MEP uniquely or commonly in ET, PV, and HD. Heme metabolism, JAK/STAT signaling, and cell cycle-related pathways were elevated in all three subtypes. TNFα-NFκB, TCR and BCR signaling pathways were attenuated in all cases. Canonical Wnt signaling was elevated specifically in ET MEPs compared to HSCs, while FCGR activation signaling was attenuated in ET MEPs. In HD MEPs, glycolysis signaling was upregulated, while TGFβ, NOTCH and VEGF signaling pathways were downregulated. By further validation, a higher expression of β-catenin was detected in MEPs compared with HSCs of JAK2V617F+ MPN mice (Figure 1A).
CD34+ HSPCs were isolated from BM of ET patients to induce MK differentiation. The culture-derived cells were used for flow cytometry and immunofluorescent staining. The percentage of CD41- , CD61- and CD42b-expressing cells were significantly reduced after culturing with 10μm XAV939 compared with control group. The percentage of CD41+ platelets were also reduced. When measuring the DNA ploidy of MK, a higher fraction of high ploidy cells (≥8N) was found in the control group than in the XAV939 group, indicating that Wnt/β-catenin inhibitor inhibits MK generation and maturation in vitro (Figure 1B-C).
Consistent with in vitro experiments, XAV939 treatment significantly reduced peripheral platelet counts and hemoglobin levels in JAK2V617F+ MPNmice (Figure 1D). BM HSPC compartment of the XAV939 treatment-effective mice exhibited a lower proportion of high-ploidy MKs (≥8N) and a higher proportion of low-ploidy MKs (<8N) compared with that of control mice. Additionally, a lower frequency of MEP subpopulation as well as MKP subpopulation within the LK (Lin- c-Kit+ Sca-1-) cells was observed in the XAV939 treatment-effective mice compared with that in the control mice, suggesting that Wnt/β-catenin inhibitor reduced platelet production by blocking MEPs and MKPs and by inhibiting MK maturation (Figure 1E).
Conclusion: This study highlights the involvement of Wnt/β-catenin signaling in modulating megakaryocytopoiesis and indicates that aberrant activation of Wnt/β-catenin signaling, which is engaged in HSC-MEP hematopoietic differentiation, contributes to ET progression. Targeting Wnt/β-catenin signaling may be a novel therapeutic approach for MPN treatment.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.