Abstract
Treatment-free remission (TFR) is successful in approximately 40-60% of CML patients who achieve a stable and deep molecular response. Relapses may occur due to the persistence of quiescent leukemic stem cells (LSC). Pioglitazone, a drug used for diabetes, is a PPAR gamma agonist and reduces STAT5 activity, and its downstream targets HIF2α and CITED2, key guardians of the quiescent LSC. Residual LSC can be gradually purged from bone marrow niches by pioglitazone.
Objectives: to evaluate the efficacy and safety of pioglitazone used with imatinib before treatment discontinuation.
Methods: EDI-PIO (from the Portuguese Estudo de Descontinuação de Imatinibe após Pioglitazona) is a prospective, single arm, phase I/II discontinuation trial. Inclusion criteria: CML in chronic phase, treated with IM for at least 3 years, with MR4.5 reported by the International Scale (IS) for 2 years. Patients received pioglitazone 30 mg/day orally for three months before IM discontinuation. After discontinuation, BCR-ABL levels were measured by real-time quantitative PCR monthly for 12 months, every two months in the second year, and then every three months. IM was re-initiated at molecular relapse (single sample with a value of >0.1% or two consecutive samples >0.01%). TFR was calculated from IM discontinuation until molecular relapse, progression, or death to causes related to CML. Overall survival (OS) was calculated from IM discontinuation until the last seen or death date by any cause. This trial is registered with Clinicaltrials.gov, NCT02852486.
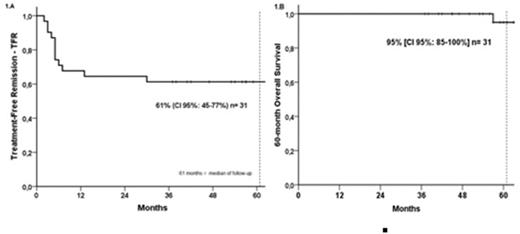
Results: Between Jun/2016 and Jan/2019, we evaluated 32 CML patients, with a median age of 54 years (29-77), treated with imatinib for a median time of 9.5 years (3-16). The median duration of MR4 and MR4.5 was 106 and 93 months, respectively. The cut-off date for this analysis was July 1st, 2022. One patient left the trial before imatinib discontinuation and was not analyzed for TFR. There were no grade 3 or 4 adverse events related to pioglitazone. The median follow-up of the 31 patients that discontinued IM was 61 months (37-69). As previously reported, 15/31(48%) patients presented symptoms related to withdrawal syndrome. Twelve patients presented molecular relapse after a median time of 5 months (2-30). Nine relapses in the first six months and three at 7, 13, and 30 months after stopping imatinib. All relapsed patients re-achieved MMR in a median time of 3 months (1.8-4.1). One patient developed an anal canal adenocarcinoma in the third year after discontinuation and was treated with surgery and chemotherapy. TFR was 71%, 67%, 61% and 61% at 6,12, 30 and 60 months (Figure 1A), respectively. 60-month OS was 95% (CI 95%: 85-100%)(Figure 1B). There were 5 cases of COVID-19 among the 19 patients in TFR (26%) and two suspects. Four cases were mild, and one patient in MR4.5 died due to severe COVID-19. Sokal score and MR4.5 duration were significant factors for prolonged TFR (P=0.032 and 0.012, respectively). Conclusions: the combination of pioglitazone and IM was feasible and safe, with TFR rates consistent with previous discontinuation trials. The 5-year long-term follow-up demonstrated durable and stable molecular responses.
Disclosures
Pagnano:Wieth/Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; GSK: Consultancy; Astellas: Consultancy, Honoraria. Delamain:Libbs Farmacêutica: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.