Abstract
Background: Calreticulin (CALR) mutations are the second most frequent genetic alteration associated with essential thrombocythemia (ET) and myelofibrosis (MF), accounting for most cases of JAK2 V617F unmutated myeloproliferative neoplasms (MPN). As opposed to JAK2 allele burden, which associates significantly with phenotypic traits, including thrombotic risk, as well as disease evolution, there is limited data regarding the prognostic impact of CALR allele burden, as measured by variant allele frequency (VAF). The objective of this study was thus to determine the phenotypic and prognostic correlates of high CALR VAF in patients with ET and MF.
Methods: This was a retrospective multicenter review (9 Quebec centers) of consecutive patients enrolled in the GQR LMC-NMP registry diagnosed with CALR mutated ET or MF as per World Health Organization 2016 criteria. Assessment of mutational status of exon 9 of the CALR gene was performed using fragment length analysis following a nucleic acid amplification test with fluorescent primers according to standard methods (Klampfl et al.N Engl J Med, 2013). A genetic analyser (ABI 3130, Life Technologies) was used to determine the size of detected insertions/deletions. Mutation burden was assessed using a quantitative assay, analytically validated to be highly reproducible and sensitive to a level of 1%. Standard statistical methods were used for analyses.
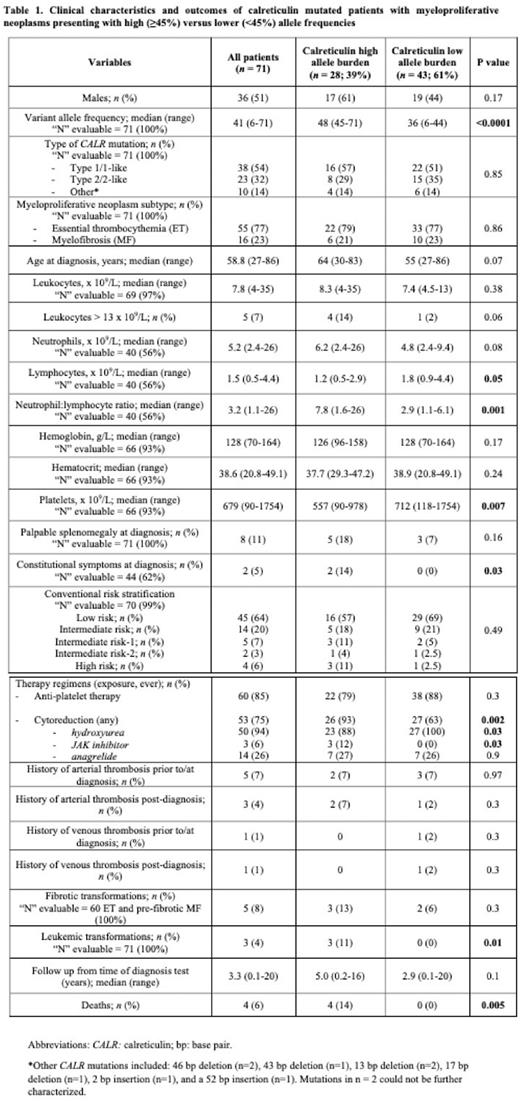
Results: Of the 71 patients studied with CALR mutated ET (n = 55) and MF (n = 16; 11 primary, 5 pre-fibrotic), median age was 58 years (range 27-86) and 36 (51%) were male (Table 1). Median VAF was 41% (range 6-71%), with few patients having VAF below 20% (n = 5, 7%) or above 50% (n = 9, 13%). ROC analysis established a VAF of 45% as the most discriminant cut-off value for survival outcome, which coincides broadly with conventional cut-offs for loss of heterozygosity (LOH) (~50%). Of the 71, 28 (39%) had a VAF ≥ 45% (median 48; range 45-71); the remainder (n = 43, 61%) had VAF < 45% (median 36; range 6-44).
Proportions of type 1 and 2 CALR mutations, MPN subtype (ET vs MF), age at MPN diagnosis, and risk stratification scores were similarly balanced among lower vs high VAF cohorts (p = 0.85, 0.86, 0.07 and 0.49, respectively). Compared to patients with lower VAF, patients with VAF ≥ 45% presented statistically significantly lower platelet counts (557 vs 712 x109/L, p = 0.007), and higher neutrophil to lymphocyte ratios (NLR; 7.8 vs 2.9, p = 0.001), with a trend towards more frequent leukocytosis > 13x109/L (14 vs 2%, p = 0.06); no significant differences in hemoglobin/hematocrit were observed (p = 0.17, 0.24 respectively). Patients with CALR VAF ≥ 45% were also significantly more likely to exhibit constitutional symptoms (14 vs 0%, p = 0.03) and require cytoreduction (93 vs 63%, p = 0.002). While thrombosis and fibrotic transformations were proportional between groups (p = 0.3), patients with VAF ≥ 45% exhibited more frequent transformation to acute leukemia (3 vs 0 events, p = 0.01; n = 2 MF, n = 1 ET) and recorded a higher number of deaths (4 vs 0, p = 0.005; n = 2 each ET/MF) over a similar follow-up period (5 vs 2.9 years, p = 0.1).
Kaplan-Meier curves stratified by high (≥ 45%) vs low (< 45%) CALR VAF revealed shorter leukemia-free (11 vs 0%, p = 0.04) and overall survival rates (14 vs 0%, p = 0.03) in patients with high CALR allele burden. There were no differences in thrombosis-free (4 vs 5%, p = 0.68) and myelofibrosis-free (13 vs 6%, p = 0.80) survival.
Subsequent CALR VAF testing was available in 15 patients (21%), disclosing minimal change in VAF over time (median 0%; range -13 to 2%), regardless of exposure to cytoreductive therapy.
Conclusion: While limited by a retrospective design and restricted sample size, this data provides a preliminary signal suggesting that CALR mutated MPN subjects (predominantly ET patients in our cohort) harboring high VAF (threshold ≥ 45%), may comprise a higher-risk subgroup, exhibiting: i) more aggressive phenotypic features, and ii) less favorable outcome patterns, particularly regarding leukemic transformation and overall survival, though total events were limited. Further validation is clearly required to confirm findings in larger datasets, though this study effectively raises the possibility that allelic burden, already known to be of importance in JAK2 mutated subjects, could potentially be considered in the individualized assessment of those that are CALR mutated.
Disclosures
Busque:Novartis: Consultancy. Szuber:Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.