Abstract
Introduction Multiple myeloma (MM) bone disease is caused by increased bone resorption due to overactivated osteoclasts coupled with impaired bone formation. Activation of osteoclasts is triggered by the production of osteoclastogenic factors secreted by multiple myeloma cells. Our previous work demonstrated that matrix metalloproteinase 13 (MMP-13) is one of the critical osteoclastogenic factors highly secreted by MM cells. Further studies indicated that checkpoint inhibitor programmed death-1 homolog (PD-1H/VISTA) functions as the MMP-13 receptor on osteoclasts and mediates MMP-13 induced osteoclast activation (Fu etc. ASH 2019, 2020, 2021). Interestingly, PD-1H associates with cytoskeletal proteins and regulates the F-actin cytoskeleton reorganization which is critical for osteoclast bone resorption activity (Fu etc. ASH 2021). However, the mechanism of PD-1H mediated MMP-13 induced osteoclast cytoskeleton regulation and MM bone disease remains unclear. Here we present data on MMP-13/PD-1H signaling triggering the reorganization of the osteoclast cytoskeleton.
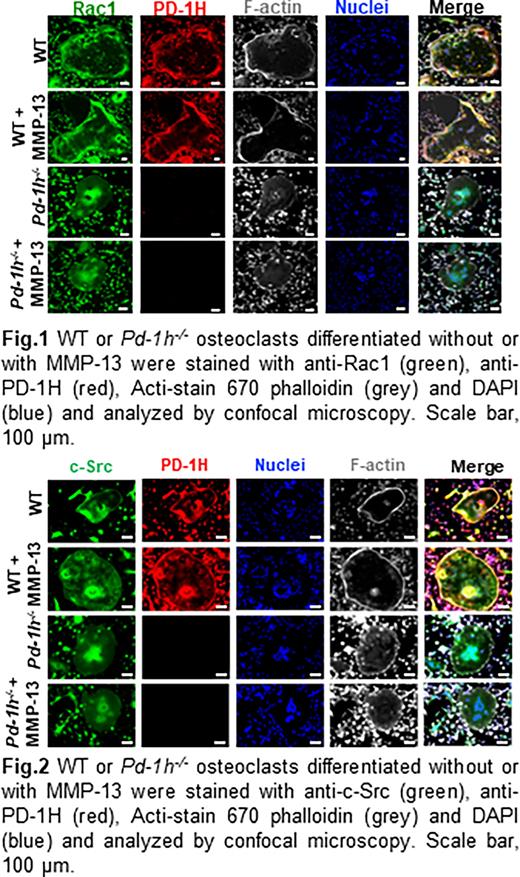
Methods and Results Rho GTPases substrates Rac1/2 are well known as the key regulators of the dynamic actin cytoskeleton rearrangements in osteoclasts (Croke M etc. 2011). To examine the mechanisms by which MMP-13/PD-1H signaling affects the reorganization of the F-actin cytoskeleton, bone marrow mononuclear cells from WT or Pd-1h-/-mice were cultured in osteoclast differentiation medium with or without MMP-13. Activated Rac1 was detected in Rac1 pull-down complex from whole cell lysates by western blotting. The results showed that MMP-13 activated Rac1 in WT osteoclasts but not Pd-1h-/- osteoclasts. Of note, Pd-1h-/-osteoclasts also displayed decreased baseline Rac1 activation relative to WT controls. Immunofluorescence staining further indicated that PD-1H and Rac1 co-localized, especially at the F-actin belt in WT osteoclasts. Consistent with the decreased Rac1 activation and F-actin belt formation observed in Pd-1h-/- osteoclasts, Rac1 failed to localize at F-actin-rich areas in Pd-1h knockout cells (Fig. 1).
Non-receptor tyrosine kinase c-Src associates with RANK and mediates RANKL-induced Rac1 activation and cytoskeleton reorganization in osteoclasts (Kuroda etc. 2012). Confocal immunofluorescence staining of the WT osteoclasts indicated that c-Src and PD-1H almost completely co-localized on the F-actin sealing belt and perinuclear area. Co-immunoprecipitation assays confirmed the direct binding of c-Src to the C-terminal intracellular domain of PD-1H as a PD-1H 1-215 mutant lacking its intracellular domain failed to bind c-Src. Immunofluorescence staining of c-Src, PD-1H and F-actin indicated that in contrast to WT osteoclasts, c-Src in Pd-1h-/- osteoclasts mainly accumulated in perinuclear areas, but not in the F-actin belt (Fig. 2). Finally, western blotting of the osteoclast lysates showed that MMP-13 induced c-Src phosphorylation/activation in WT osteoclasts, which was impaired in Pd-1h-/- cells.
Conclusions Taken together, this study reveals the novel role of MMP-13/PD-1H signaling in the regulation of cytoskeleton reorganization in osteoclasts, a critical process to ensure osteoclast bone resorption activity. PD-1H directly associates with c-Src, a key regulator of cytoskeleton reorganization where it localizes to the sealing zone in mature osteoclasts and regulates podosome dynamics by activating Rac1. We found that MMP-13 promotes c-Src/Rac1 signaling activation and subsequently promotes osteoclast bone resorption activities via a PD-1H/VISTA-dependent mechanism. This study hence revealed a novel role of the checkpoint inhibitor PD-1H/VISTA in osteoclast cytoskeleton regulation and subsequently multiple myeloma bone disease.
Disclosures
Mapara:Ossium: Consultancy. Lentzsch:Takeda: Consultancy; Nataea: Consultancy; Peerview: Speakers Bureau; Zentalis: Research Funding; Caelum Bioscience: Consultancy, Other: Stock ownership; GlaxoSmithKline: Consultancy; Magenta: Other: Equity Ownership; Janssen: Consultancy; Poseida: Other: Equity ownership; Clinical Care Options: Speakers Bureau; Prothena: Honoraria; Sanofi: Consultancy, Research Funding; Oncopeptides: Consultancy; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.