Abstract
Advances in conditioning, graft-versus-host disease (GVHD) prophylaxis and antimicrobial prophylaxis have improved the safety of allogeneic hematopoietic cell transplantation (HCT), leading to a substantial increase in the number of patients transplanted each year. This influx of patients along with progress in remission-inducing and posttransplant maintenance strategies for hematologic malignancies has led to new GVHD risk factors and high-risk groups: HLA-mismatched related (haplo) and unrelated (MMUD) donors; older recipient age; posttransplant maintenance; prior checkpoint inhibitor and autologous HCT exposure; and patients with benign hematologic disorders. Along with the changing transplant population, the field of HCT has dramatically shifted in the past decade because of the widespread adoption of posttransplantation cyclophosphamide (PTCy), which has increased the use of HLA-mismatched related donors to levels comparable to HLA-matched related donors. Its success has led investigators to explore PTCy’s utility for HLA-matched HCT, where we predict it will be embraced as well. Additionally, combinations of promising new agents for GVHD prophylaxis such as abatacept and JAK inhibitors with PTCy inspire hope for an even safer transplant platform. Using 3 illustrative cases, we review our current approach to transplantation of patients at high risk of GVHD using our modern armamentarium.
Introduction
We are living in an era of remarkable progress and change in the field of allogeneic hematopoietic cell transplantation (HCT). Not only does nonrelapse mortality (NRM) continue to decline with modern conditioning, graft-versus-host disease (GVHD), and antimicrobial strategies, but the success of posttransplant cyclophosphamide (PTCy) has led to the widespread use of HLA-haploidentical HCT (haplo-HCT), greatly expanding the donor pool.1-5 With advances in both transplantation techniques and hematologic oncology, new challenges have been identified in the prevention and management of GVHD. Reduced intensity conditioning (RIC) regimens and safer GVHD prevention strategies have led to transplantation of patients who are older; possess more comorbidities; are more heavily pretreated; and are overall at higher risk for transplant-associated complications. The success of first-degree relative haplo-HCT with PTCy has increased interest in second-degree relative HLA haploidentical donors and HLA-mismatched unrelated donors (MMUD), leading to questions on how to best mitigate the GVHD and NRM risk associated with these alternative donor sources.6,7 The use of molecularly targeted and immune-based therapies for hematologic malignancies in the peritransplant setting has altered the risk of GVHD and other transplant-associated complications. Finally, HCT is being increasingly used to treat patients with benign hematologic disorders and primary immunodeficiencies, patient populations that do not benefit from the graft-versus-malignancy effect or any degree of GVHD. These challenges have increased the number of patients at high risk of GVHD, and new approaches must be identified to mitigate this risk and improve the safety of this therapy.
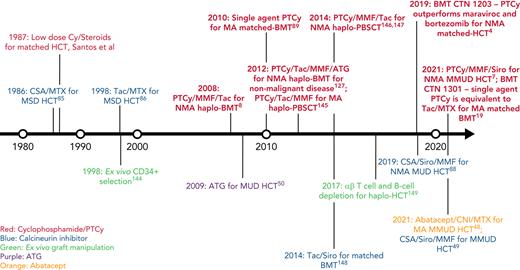
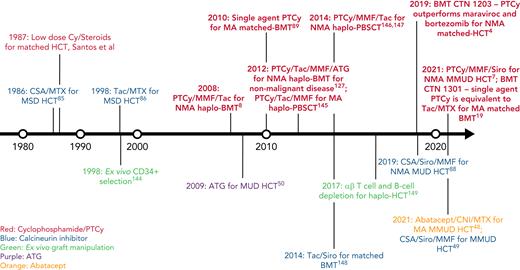
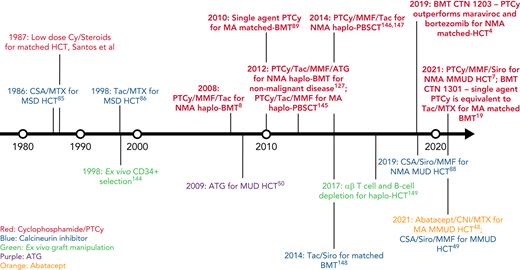
In this review, we discuss our approach to managing patients at high risk for GVHD in the context of 3 informative cases. Given the significant body of data on haplo-HCT with PTCy using a first-degree relative, we focus on GVHD prophylaxis strategies for MMUD HCT; matched unrelated donor (MUD) HCT; and haplo-HCT with a second-degree relative. Additionally, we will discuss our approach to managing patients needing to cease immunosuppression before day +100; those receiving a posttransplant maintenance therapy; elderly patients, particularly those aged ≥60 years; those receiving an immune checkpoint inhibitor or autologous hematopoietic cell transplant before HCT; and those undergoing transplantation for a nonmalignant disorder. For a historical overview of major GVHD prophylaxis regimens for patients at high risk for GVHD, see Figure 1.
Timeline of major GVHD prophylaxis strategies for high-risk patients undergoing allogeneic hematopoietic cell transplantation.50 ,144-149 CSA, cyclosporin; Cy, cyclophosphamide; haplo-BMT, HLA-haploidentical bone marrow transplantation; MSD, matched sibling donor; PBSCT, peripheral blood stem cell transplantation; Siro, sirolimus; Tac, tacrolimus.
Timeline of major GVHD prophylaxis strategies for high-risk patients undergoing allogeneic hematopoietic cell transplantation.50 ,144-149 CSA, cyclosporin; Cy, cyclophosphamide; haplo-BMT, HLA-haploidentical bone marrow transplantation; MSD, matched sibling donor; PBSCT, peripheral blood stem cell transplantation; Siro, sirolimus; Tac, tacrolimus.
Case 1
A 68-year-old African American female with FLT3-internal tandem duplication (ITD) mutant acute myeloid leukemia (AML) had persistent measurable residual disease via FLT3-ITD polymerase chain reaction despite induction chemotherapy with daunorubicin, cytarabine, and midostaurin and 1 cycle of consolidative high-dose cytarabine plus midostaurin and was referred for consideration for HCT. In terms of her immediate family, both parents were deceased, and she had no children or siblings. Three cousins were HLA typed and found to be disparate to the patient. An unrelated donor search revealed no HLA-matched donors but 1 donor with a 9/10 HLA. The patient proceeded with a peripheral blood HCT with nonmyeloablative (NMA) conditioning per Luznik et al8 and posttransplant immunosuppression consisting of PTCy, mycophenolate mofetil (MMF), and sirolimus as per Kasamon et al.9 Although she achieved neutrophil and platelet engraftment on days +24 and +27, her hemoglobin and platelet count remained persistently low through day +90, hovering around 8 g/dL and 40 000/μL. A day +90, bone marrow biopsy revealed no morphologic evidence of leukemia; persistence of the FLT3-ITD mutation at a variant allele frequency of 1.1% and 99% donor chimerism. Peripheral blood at day +90 showed 96% donor engraftment of the CD3+ cells and 94% engraftment of the donor CD34+ cells. Sirolimus was stopped and sorafenib 200 mg daily was started. Counts improved and day +180 and day +360 bone marrow biopsies reported morphologic remission without presence of the FLT3-ITD mutation and 100% donor engraftment in the CD3+ and CD33+ compartments. The patient currently remains in a minimal residual disease-negative remission 16 months after transplantation and continues on maintenance sorafenib with mild gastrointestinal side effects. She will continue the sorafenib for at least 2 years posttransplant.
Case 1 discussion
Patient 1 is at high risk for GVHD because of the use of a MMUD graft, age of 68 years, and her need for early cessation of immunosuppression to facilitate improvement in persistent mild cytopenias and allow early integration of posttransplant maintenance. Additionally, the association between posttransplant maintenance therapy with sorafenib and GVHD risk is controversial.
Discovery of PTCy and associated toxicities
The probability of finding an HLA-matched donor in the US Be the Match registry is much lower for African American patients (19%) compared with patients of White European descent (75%).10 This probability, however, increases to 76% for African American patients if 7/8 HLA-matched donors are included.10 Thus, African American patients and other ethnic minorities are more likely to be transplanted if MMUDs are considered, although this donor source was traditionally associated with worse survival and GVHD outcomes when compared with HLA-matched donors in the context of tacrolimus and methotrexate-based GVHD prophylaxis.10-15 To improve outcomes with HLA-mismatched HCT, the Hopkins group discovered that they could induce tolerance of an HLA-mismatched graft by administering PTCy.8,16 Several studies have now demonstrated that survival with haplo-HCT with PTCy is similar to that after HLA-matched HCT with traditional GVHD prophylaxis.2,3,5,17,18 For more information on immune reconstitution and the risk of relapse after PTCy, refer to the following cited manuscripts.4,19,20
Several unique toxicities have been documented after PTCy-based HCT (supplemental Table 1, available on the Blood Web site). After PTCy-based haplo-HCT using a peripheral blood graft, fever and cytokine release syndrome have been documented as early adverse effects often occurring before day +10, although these are felt to be related to the intense alloreactivity occurring as a result of the HLA mismatch between donor and recipient or use of myeloablative conditioning.21-23 Given its effects on T-cell reconstitution, PTCy-based HCT has been shown to be associated with an increased incidence of cytomegalovirus infections compared with a calcineurin inhibitor (CNI)-based HCT, although this increased incidence of cytomegalovirus infections has not led to worse survival outcomes compared with other GVHD prophylaxis strategies in randomized clinical trials.19,24-26 Although PTCy-based HCT has been associated with significant rates of hemorrhagic cystitis, this risk can be effectively mitigated with the use of infusional sodium 2-mercaptoethanesulfonate.27-30 Although cyclophosphamide has been historically associated with increased rates of cardiac toxicity, results are inconclusive as to whether PTCy-based HCT is associated with an increased rate of cardiac toxicity.31-33 Finally, in a retrospective study of PTCy-based HCT, rates of transplant-associated thrombotic microangiopathy were low and similar to non-PTCy-based HCT.34,35 Overall, PTCy-based HCT has an acceptable toxicity profile, and adverse events can be mitigated with close monitoring and appropriate preventative measures. An early-phase clinical trial is currently under way evaluating whether a reduced dose of PTCy can effectively mitigate associated toxicities and provide a similar level of GVHD prevention.36
Modern approaches to GVHD prophylaxis for MMUD HCT
Given the success of PTCy in haplo-HCT, PTCy was further evaluating in other donor sources such as MMUDs.7,9,37-39 The National Donor Marrow Program sponsored a phase 2 trial evaluating PTCy, sirolimus, and MMF for GVHD prophylaxis in 80 patients undergoing MMUD-HCT with a bone marrow graft.7 Patients receiving myeloablative (MA) conditioning had a grade 3-4 acute GVHD (aGVHD) rate of 18% at day +100, a chronic GVHD (cGVHD) rate of 36% at 1 year, and an NRM rate of 8% at 1 year, whereas those receiving RIC had rates of 0%, 18%, and 10%, respectively.7
With survival after HLA-mismatched HCT with PTCy rivaling that after HLA-matched HCT, many wonder whether PTCy has nullified the detrimental effect of HLA mismatch.40-45 As such, the association of HLA-mismatched with GVHD has been examined in patients receiving haplo-HCT with PTCy where class II mismatching at HLA-DRB1 and HLA-DPB1 reduced relapse without increasing GVHD.46 Additionally, HLA-C mismatch in the graft-versus-host direction was associated with an increased risk of cGVHD. Although these results are very exciting, further prospective studies are needed to assess the impact of individual HLA mismatches on GVHD.
Other advances in HLA-mismatched HCT include abatacept, a modified antibody inhibiting the T-cell costimulatory axis of CD28-CD80/86, and triple immunosuppression with tacrolimus, sirolimus, and MMF.47 A recent phase 2 trial demonstrated that abatacept, a CNI, and methotrexate (MTX) was associated with reduced aGVHD and NRM in MMUD-HCT patients when compared with a historical cohort receiving a CNI and MTX alone.48 Additionally, a phase 1/2 trial of triple immunosuppression with tacrolimus, sirolimus, and MMF for MMUD-HCT reported improved aGVHD, NRM, and overall survival (OS) but similar cGVHD in comparison with patients on a previous study of cyclosporine and MMF for MMUD HCT.49 Although modification may be needed to reduce cGVHD rates, these 2 regimens exhibit promising results in terms of aGVHD and NRM prevention, and further study is warranted.
Given that antithymocyte globulin (ATG) has been previously reported to effectively prevent GVHD in MUD and MMUD HCT, several groups have compared outcomes between ATG and PTCy in MMUD-HCT.50-54 These retrospective studies have consistently demonstrated reductions in GVHD and improved survival outcomes with PTCy.52-54 Based on these studies, our preference is to use PTCy, MMF, and a CNI for GVHD prophylaxis for MMUD HCT. However, comparative studies of abatacept, ATG, and PTCy for MMUD HCT are needed, and studies combining abatacept or JAK inhibition and PTCy are currently under way.55,56
Elderly patients undergoing HCT
Patient age is an important risk factor for HCT and negatively affects outcomes.57,58 However, PTCy may represent a beneficial prophylaxis strategy for older patients given its association with low GVHD and NRM rates. In the era of RIC haplo-HCT with PTCy, Kasamon et al evaluated outcomes in patients aged 50 to 75 years and reported that age was not associated with grade 3-4 aGVHD, cGVHD, NRM, relapse, relapse-free survival, or OS.59 Additional reports demonstrate haplo-HCT with PTCy to be feasible in patients aged >60 or even 70 years.60,61 Several studies have reported no difference in NRM or OS between haplo-HCT with PTCy and HLA-matched HCT with traditional GVHD prophylaxis aged ≥60 years.62-64 In contrast, 2 studies have reported improved OS and reduced relapse associated with HLA-matched HCT, although HLA-matched HCT patients in these studies had higher percentages of peripheral blood grafts, which is associated with reduced relapse compared with bone marrow grafts.65-67 Many of these studies report lower incidences of aGVHD and cGVHD associated with PTCy-based haplo-HCT.63-66 Overall, RIC HCT with PTCy may represent a feasible strategy to limit NRM and GVHD in patients 60 years of age or older, but further prospective studies are needed.
Posttransplant immunosuppression duration
Several early studies attempted to define the ideal duration for post-HCT cyclosporin but provide conflicting results as to whether shorter or longer courses led to reduced GVHD and/or mortality.68-72 In light of PTCy’s ability to reduce the incidence of GVHD and NRM, the issue of duration of immunosuppression has been revisited. In prospective, single-center trials, patients receiving an NMA haplo-HCT with a bone marrow graft (n = 105) or peripheral blood graft (n = 117) were prespecified to stop tacrolimus at day +90 or day +60 if they had ≥5% donor T cells, no relapse, and no grade 2-4 aGVHD or significant cGVHD.73,74 In both studies, GVHD and NRM rates of the patients stopping immunosuppression early were highly comparable to historical cohorts of patients stopping immunosuppression at day +180.73,74 Although these data are limited, given our clinical experience, we often stop CNI immunosuppression as early as day +60 after a bone marrow transplant and day +90 after a peripheral blood transplant in patients without evidence of GVHD to limit associated toxicities or integrate posttransplantation maintenance therapeutics, similar to the patient in case 1.
Posttransplant maintenance therapies
As data emerge on posttransplant maintenance strategies, questions also arise as to each agent’s potential to exacerbate GVHD or other posttransplant complications.75,76 Although the phase 2 Radius trial reported no increased aGVHD or cGVHD associated with posttransplant midostaurin, the SORMAIN trial did report higher rates of aGVHD (24% vs 18.2%) and cGVHD (62% vs 46%) associated with posttransplant sorafenib, although these differences were not statistically compared.77,78 A phase 3 study (BMT CTN 1506) evaluating gilteritinib for posttransplant maintenance for FLT3-mutated AML patients has completed accrual, and we are awaiting the final results. However, based on clinical experience, gilteritinib does not appear to exacerbate GVHD. A phase 3 study of posttransplant maintenance with azacitidine for high-risk patients with AML and myelodysplastic syndrome (MDS) reported no difference in aGVHD or cGVHD.79 In retrospective studies of patients with chronic myeloid leukemia and Philadelphia chromosome positive B-cell acute lymphoblastic leukemia, posttransplant use of a BCR-ABL targeted tyrosine kinase inhibitor was not associated with an increase in cGVHD or TRM.80-82 Blinatumomab, IDH inhibitors, and ibrutinib are also currently being evaluated.83,84 Overall, posttransplant maintenance remains a nascent field, and further data are needed to understand the effects of these therapies on GVHD risk.
Case 2
A 33-year-old White woman with relapsed classical Hodgkin lymphoma (HL) after treatment with Adriamycin, bleomycin, vinblastine, and dacarbazine; brentuximab vedotin, and carmustine, etoposide, cytarabine, and melphalan autologous hematopoietic stem cell transplant (auto-HCT) was treated with single-agent nivolumab and achieved a complete remission after 2 cycles. She was subsequently referred for consolidative HCT. Regarding the patient’s family, she was adopted and had no knowledge of her immediate or extended biological family. A MMUD search was initiated and 2 10/10 donors were found. The patient proceeded with an NMA MUD HCT using a bone marrow graft. For GVHD prophylaxis, she received PTCy followed by MMF and tacrolimus. Her course was complicated by methicillin-resistant Staphylococcus aureus bacteremia, and she achieved neutrophil and platelet engraftment on days +19 and +24, respectively. On day +35, her MMF was discontinued. On day +56, she developed grade 2 aGVHD of the skin and lower gastrointestinal tract, which resolved with topical and systemic steroids that were tapered off by day +90. Chimerism evaluations at day +90, +180, and +360 report 100% donor engraftment of the CD3+ and CD33+ compartments. The patient is now 21 months from her HCT and remains in complete remission.
Case 2 discussion
This patient is at high risk for GVHD given her use of checkpoint inhibition before transplantation and her heavy pretreatment, including a prior autologous HCT. Additionally, given that she received a MUD HCT, she has several options for GVHD prophylaxis.
Modern approaches to GVHD prophylaxis for MUD HCT
Historically, a CNI with MTX represents the most commonly used regimen for GVHD prophylaxis in patients receiving a MUD HCT, although the rates of aGVHD and cGVHD associated with this regimen range between 30% to 50% and 30% to 70%, respectively.85-87 In an attempt to improve on these results, BMT CTN 1203 treated patients undergoing a RIC HLA-matched HCT with tacrolimus, MMF, and PTCy; tacrolimus, MTX, and bortezomib; or tacrolimus, MTX, and maraviroc and compared their outcomes to a historical cohort of patients receiving CNI and MTX.4 Of the 3 study regimens, only tacrolimus/MMF/PTCy outperformed CNI/MTX in terms of improved GVHD-free, relapse-free survival, reduced grade 3-4 aGVHD, and reduced cGVHD requiring immunosuppression. PTCy/MMF/tacrolimus and tacrolimus/MTX are now being compared in a phase 3 trial of patients undergoing RIC HCT with a peripheral blood graft (BMT CTN 1703). Apart from PTCy, a phase 3 trial published in 2019 compared cyclosporine/MMF with cyclosporine/MMF/sirolimus in RIC MUD HCT and reported lower rates of grade II-IV aGVHD and NRM along with improved OS.88 Based on these data, we consider tacrolimus/MMF/PTCy or cyclosporine/MMF/sirolimus for patients undergoing RIC MUD HCT at high risk for GVHD, although we eagerly await future comparisons between the 2 regimens.
In the setting of MA MUD HCT with bone marrow grafts, PTCy as a single agent has also demonstrated favorable GVHD and survival outcomes.19,89-91 In a recently published phase 3 trial, single-agent PTCy and ex vivo CD34+ selection, 2 calcineurin-free GVHD prophylaxis regimens, were compared with tacrolimus/MTX for patients with acute leukemia or MDS undergoing MA MUD or matched-related HCT.19 Bone marrow grafts were used with PTCy and tacrolimus/MTX, whereas peripheral blood grafts were used with ex vivo CD34+ selection. Compared with tacrolimus/MTX, single-agent PTCy had a nonsignificant higher rate of cGVHD-free, relapse-free survival, and a nonsignificant lower rate of cGVHD. Based on these findings, single-agent PTCy is a viable regimen to consider for prophylaxis of patients undergoing MA MUD HCT with a bone marrow graft, although we prefer to combine a CNI with PTCy to further reduce severe aGVHD and cGVHD rates.19,89,91-93 In studies evaluating peripheral blood transplantation, single-agent PTCy has been associated with high rates of GVHD, and additional immunosuppressive agents are needed to mitigate this risk.94,95 If aGVHD does develop after single-agent PTCy, it can be rapidly progressive and may require a combination of steroids plus a CNI for treatment.96
JAK inhibition also represents an emerging strategy for GVHD prophylaxis, with promising data seen in small, initial studies of patients transplanted for myelofibrosis and acute leukemia.56,97-100 Phase 1 clinical trials are evaluating both ruxolitinib and the JAK1-specific inhibitor itacitinib in the peritransplant setting for GVHD and cytokine release syndrome prophylaxis (NCT03320642, NCT03755414).56,100 Given their promise for primary GVHD prevention, JAK inhibitors may eventually reduce or even replace the use of traditional postgrafting immunosuppressants such as CNIs and MMF.
Pretransplant checkpoint inhibition and auto-HCT as risk factors for GVHD and prophylaxis strategies
Initial studies evaluating transplant outcomes after pretransplant use of CPI reported higher than expected rates of grade 3-4 aGVHD (17%-29%) and GVHD-related mortality (9%-35%).73,101-103 Based on these data, HCT after CPI was felt to be risky, and the US Food and Drug Administration added a warning and precautions statement for complications after HCT in the prescribing information for nivolumab.104 In contrast, several studies demonstrated that PTCy may effectively mitigate the excess GVHD risk associated with pretransplant CPI use. Among patients with myeloid malignancies, a retrospective study of patients receiving CPI before HCT for AML or MDS reported a trend toward reduced grade 3-4 aGVHD and 1-year NRM for patients receiving PTCy vs not.105 Two retrospective studies compared outcomes of patients with HL receiving vs not receiving CPI before NMA haplo-HCT with PTCy and reported similar rates of aGVHD, cGVHD, NRM, and OS.106,107 In the largest and most recent of these studies, Merryman et al retrospectively evaluated 209 patients with classical HL receiving HCT after checkpoint blockade.108 In comparison with patients receiving non-PTCy-based GVHD prophylaxis, those receiving PTCy had improved GVHD-free, relapse-free survival, and reduced cGVHD.108 Additionally, the patients receiving a HCT with PTCy had improved OS compared with those receiving a non-PTCy regimen.108 Based on these impressive data, we favor use of a PTCy-based GVHD prophylaxis regimen for patients undergoing HLA-matched HCT after treatment with a CPI.
Auto-HCT before an HCT may also be associated with a higher risk of NRM and GVHD.109,110 However, this risk is mitigated by using RIC or NMA conditioning rather than MA.111-114 With the advent of PTCy, a European group performed a retrospective analysis comparing outcomes between patients receiving matched-donor HCT vs haplo-HCT with PTCy to treat HL relapsing after auto-HCT.115 The matched donor HCT recipients and haplo-HCT recipients had similar rates of grade 2-4 aGVHD and grade 3-4 aGVHD, whereas the haplo-HCT recipients had a much lower rate of moderate-to-severe cGVHD.115 Based on these studies, we would recommend RIC for patients undergoing HCT after auto-HCT and believe that PTCy is a very valuable strategy to further reduce cGVHD in this population.
Bone marrow vs peripheral blood grafts for PTCy-based HCT
Among patients receiving PTCy for GVHD prophylaxis, 3 registry-based studies have compared outcomes between patients receiving a bone marrow or peripheral blood graft, with increases in aGVHD and/or cGVHD demonstrated with the latter.67,116,117 Im et al further explored this question in the context of conditioning intensity.117 When patients were divided into MA and RIC groups and analyzed separately, graft source was not associated with aGVHD.117 However, in patients receiving RIC, peripheral blood grafts were associated with an increase in cGVHD.117 Although none of the studies reported an association between graft source and OS, Bashey et al reported a lower rate of relapse with peripheral blood grafts.67 However, relapse reduction has not been demonstrated in lymphoma patients, and thus we used a bone marrow graft for case 2.
Haploidentical vs matched donors for HL
Several studies have compared haplo vs HLA-matched HCT for HL. In the first of these studies by Burrows et al, haplo-HCT was associated reduced NRM, improved progression-free survival, and reduced relapse when compared with HLA-matched HCT.118 In a retrospective analysis of 198 patients with HL, haplo-HCT vs an HLA-matched HCT was associated with improved relapse-free survival and a reduction of relapse.119 No statistically significant differences in GVHD were noted for either study.118,119 Additionally, only the haplo-HCT patients received PTCy in both studies.118,119 Although increased alloreactivity and an improved graft-versus-malignancy effect may explain the improvement in survival outcomes associated with haplo-HCT, PTCy (and not donor source) may also explain the improved survival outcomes given its efficacy at preventing GVHD, particularly in a heavily CPI-treated population. Further prospective studies are needed to tease out this distinction.
Case 3
A 21-year-old African American male with sickle cell disease (hemoglobin SS) initiated treatment with hydroxyurea at age 12 years because of recurrent vaso-occlusive crises. Despite excellent adherence with hydroxyurea, he suffered from 5 episodes of acute chest syndrome requiring intensive care unit admission over 7 years. After several conversations with his longitudinal hematologist, the HCT team, and his family, the patient decided to proceed with HCT.
The patient’s immediate family consisted of a 47-year-old father and a 46-year-old mother. The patient had no siblings but did have 2 aunts and 1 uncle, aged 33, 34, and 38 years, respectively. His 34-year-old aunt and 38-year-old uncle possessed sickle cell trait. HLA typing revealed that his father, mother, 33-year-old aunt, and 38-year-old uncle all shared 1 HLA haplotype. Class 1 and 2 screens for donor-specific antibodies were negative. The patient proceeded with NMA, haploidentical bone marrow transplantation from his 33-year-old aunt. He received rabbit ATG 0.5 mg/kg on days −9 to −7; fludarabine on days −6 to −2; cyclophosphamide on days −6 and −5; and 400 cGy of total body irradiation on day −1.120 The patient received cyclophosphamide on days +3 and +4 in addition to tacrolimus and MMF starting on day +5. His transplant course was complicated by a pain crisis starting on day −7 during the conditioning period. He achieved neutrophil and platelet engraftment on days +25 and +31, respectively. Donor bone marrow myeloid chimerism was 66% and 73% at day +90 and day +180, respectively. Two years posttransplant, he remains pain free off of hydroxyurea and opiates and has not experienced any vaso-occlusive crises, episodes of acute coronary syndrome, or other sickle cell-related complications.
Case 3 discussion
Given that the patient is being transplanted for a benign hematologic disorder, he does not benefit from the graft-versus-malignancy effect, thus the goal of reducing GVHD is paramount. Additionally, second-degree relative HLA-haploidentical donors represent an emerging donor source for haplo-HCT, and their association with clinical outcomes has not been characterized until relatively recently.
Haplo-HCT with PTCy for benign hematologic disorders and primary immunodeficiencies
Despite favorable outcomes with HLA-matched HCT, many sickle cell and hemoglobinopathy patients, including those of African descent, lack access to matched donors.10,121-126 With the advent of PTCy as viable strategy to prevent GVHD and facilitate HLA-mismatched transplantation, several studies subsequently evaluated the use of haplo-HCT with PTCy in patients with sickle cell disease.120,127-131 Bolaños-Meade et al reported low rates of GVHD and NRM with the use of haplo-HCT with a bone marrow graft; ATG, fludarabine, cyclophosphamide, and 200 cGy total body irradiation (TBI) for conditioning; and PTCy/MMF/CNI for GVHD prophylaxis.127 A follow-up study reported that increasing the TBI from 200 to 400 cGy improved engraftment rates from 78% to 94%, with a 29% rate of aGVHD, an 18% rate of cGVHD, and 100% OS.120 Additional studies used alemtuzumab and low-dose TBI or thiotepa-based regimens for conditioning and reported promising results.128,130 PTCy has also been shown to be a feasible strategy for GVHD prophylaxis in primary immunodeficiencies other benign hematologic disorders, including aplastic anemia, Fanconi anemia, and thalassemia.120,132-139 Among patients with relapsed/refractory severe aplastic anemia, Arcuri et al reported that increasing the conditioning dose of cyclophosphamide from 14.5 mg/kg per day to 25 mg/kg per day and/or increasing the TBI dose above 200 cGy led to improvements in event-free survival.139 In the field of primary immunodeficiencies, Dimitrova et al transplanted 20 such patients using a radiation-free, serotherapy-free RIC regimen with PTCy, sirolimus, and MMF and reported a low incidence of aGVHD, no cases of cGVHD, and a 1-year OS rate of 90%.135 Although we believe that haplo-HCT with PTCy is an important strategy to facilitate access to transplantation for patients with benign hematologic disorders and primary immune deficiencies, further research is needed to define the best regimen that facilitates engraftment and limits NRM.
HLA-mismatched HCT with non–first-degree relatives
Most of the haplo-HCT literature used first degree relatives as donors, but if second- or greater-degree HLA-haploidentical relatives could also be used, this could expand the donor pool further, particularly for patients who lack an HLA-haploidentical first-degree relative donor. Given the negative association between donor age and outcomes, use of second-degree relative haploidentical donors could also allow preferential selection of younger donors, particularly if recipients only had older first-degree relative haploidentical donors.57,140,141 A study combining data from a prospective clinical trial (n = 15) and a retrospectively treated cohort (n = 18) reported similar outcomes after transplanting patients using HLA-haploidentical second- or third-degree relative HCT compared with historical data for first-degree relative haplo-HCT.6 Our institutional preference is to pick the youngest donor when multiple first- and/or second-degree haploidentical donors are available, but more studies are needed.57,140,141
Future directions: GVHD biomarkers
Although they are not in routine clinical use, biomarkers have shown promise as another method to identify patients at high risk of GVHD early after HCT. Multiple protein biomarkers have been demonstrated to predict of outcomes in patients diagnosed with GVHD, but need further validation in terms of their ability to predict GVHD development.142 In the setting of PTCy-based HCT, day +30 levels of interleukin-2Rα, tumor necrosis factor-1, ST2, and REG3α predicted NRM, and day +30 interleukin-2Rα level was associated with future development of aGVHD.143 Additionally, in our report of patients undergoing HCT with PTCy, combined elevations in effector CD4+ conventional T cells and soluble CXCL9 at day +28 were predictive of the future development of aGVHD.20 Although further study is warranted to validate these findings, predictive biomarkers may allow physicians to adjust the duration of a patient’s GVHD prophylaxis regimen and further improve risk-adapted strategies for HCT recipients.
Conclusions
PTCy has not only expanded the donor pool by facilitating haplo-HCT, it has also proven to be an effective strategy to prevent GVHD in patients undergoing MMUD HCT; age ≥60 years; needing to stop immunosuppression before day +100; receiving CPI or auto-HCT before HCT; and undergoing HCT for a nonmalignant condition. We anticipate that with further study, the implementation of PTCy will expand to conventional allografting using HLA-matched donors. Additionally, several novel approaches have the potential to further reduce GVHD and NRM, such as abatacept, JAK inhibitors, or biomarker-driven adaptive strategies. As such, we expect that GVHD prophylaxis will continue to evolve and improve outcomes for a wider population of HCT recipients.
Acknowledgment
This work was supported by a grant from the National Institutes of Health/National Cancer Institute (P01CA15396).
Authorship
Contribution: J.R., S.R.M., and L.L. conceived and wrote the manuscript; and all authors approve of this version.
Conflict-of-interest disclosure: J.R. owns financial stock in Merck. S.R.M. receives grant/research/clinical trial support from Gilead Sciences, Aprea Therapeutics, and the McCabe Fund. L.L. holds a patent with WindMiL therapeutics; receives grant/research/clinical trial support from Genentech; and is a consultant/advisory board member for Gilead Sciences, Rubius Therapeutics, Precision Biosciences, and Talaris Therapeutics.
Correspondence: Leo Luznik, Division of Hematologic Malignancies, Department of Oncology, Johns Hopkins University School of Medicine, 1650 Orleans St, Cancer Research Building 1, Room 2M08, Baltimore, MD 21287; e-mail: luznile@jhmi.edu.
References
Author notes
The online version of this article contains a data supplement.