In this issue of Blood, Karamatic Crew et al examined alloantibody data from different patients with similar immunohematologic reaction patterns and identified PIEZO1 as the common but previously unidentified genetic carrier of the corresponding antigens.1 With the detection of at least one natural human alloantibody directed against an erythrocyte antigen and the description of the causative DNA polymorphism on a gene different from all other genes encoding antigens of existing blood group systems, the core criteria for the recognition of a candidate new blood group system have been fulfilled.
The discovery of such a “new” blood group system is preceded by years, often decades, of work, as in this case with the first Er antigen, named after the first propositus, reported in 1982.2 During this period of time, comparative immunohematologic methods are used to meticulously collect individual samples, sometimes from all over the world, and the initial information is assembled into a first mosaic of what may then become a coherent picture of a new blood group system. Blood group experts with their deep-freeze archives had only been waiting for new methods, eg, whole-exome sequencing, to finally identify these cryptic blood groups. A total of 13 new blood group systems, from FORS (ISBT 031) in 2012 to ABCC1 (ISBT 043) in 2020 have been described and officially ratified by the International Society of Blood Transfusion (ISBT) Working Party for Red Cell Immunogenetics and Blood Group Terminology. the latest Working Party report is in revision and expected to be published in Vox Sanguinis in 2022.3-5 Using immunoprecipitation, whole-exome sequencing, CRISPR/Cas9–mediated gene knockout and expression studies in an erythroblast cell line and pending the ratification of the ISBT Terminology Working Party, Karamatic Crew et al have once again6 succeeded in the description of a candidate new blood group system, the Er system, proposed tentatively as ISBT 044, encoded by PIEZO1.1
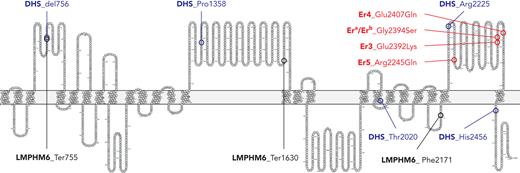
The timing for the description of PIEZO1 as the carrier of the genetic information for the blood group system Er could not have been better. On 4 October 2021, presumably just when the authors were compiling their data at the International Blood Group Reference Laboratory in Bristol, United Kingdom, in Stockholm, Ardem Patapoutian was announced as the 2021 Nobel Laureate in Medicine and Physiology for his discoveries involving the two mechanically activated ion channels Piezo1 and Piezo2. Similarly to Piezo crystals, in which electrical charges are generated under mechanical deformation, the transmembrane Piezo proteins translate mechanical forces into the flow of ions through cell membranes.7 Such cellular mechanotransduction plays important physiologic roles in somatosensation (touch perception, proprioception, and pulmonary respiration), red blood cell volume regulation, vascular physiology, and various human genetic disorders.8 It almost seems that whenever in the course of evolution mechanical pressure was needed to be measured physiologically, Piezo1 and Piezo2 were utilized. Accordingly, these proteins not only have a wide variety of biologic functions but also are linked to a number of hereditary diseases. The genetic PIEZO1 disorders include dehydrated hereditary stomatocytosis (DHS1; 194380) and lymphatic malformation 6 (LMPHM6; 616843). DHS1 with and without pseudohyperkalemia is frequently observed to be caused by dominant gain-of-function mutations.9 LMPHM6, on the other hand, appears to follow a recessive mode of inheritance caused by compound heterozygosity for alleles with absent protein expression.10 At this point, the protein variants that cause known hereditary diseases and the blood group antigens newly localized to PIEZO1 meet and may be considered from 2 different points of view: disease-associated genetic disorder or blood group (see figure). It would be worthwhile to study some of the patients affected by DHS1 or LMPHM6 to determine whether they express new Er antigens. Conversely, are different Er antigens associated with particular diseases? This idea was briefly, but certainly not conclusively, addressed within this article. Be that as it may, Piezo1 is a remarkable protein. This single protein teaches us to look beyond our respective horizons, to dive into foreign disciplines and learn new things.
Piezo1 protein (Uniprot Q92508) amino acids 602 to 2521 and known mutations in PIEZO1 encoding amino acid substitutions. Er blood group antigens encoded by PIEZO1 are given in red and as detailed by Karamatic Crew et al.1 Gly2394 is required for expression of the high-prevalence antigen Era, whereas Ser2394 encodes the antithetical low-prevalence antigen Erb. Proposed novel high-prevalence antigens Er4 and Er5 are associated, respectively, with Gln2407 and Arg2245 in Piezo1. One Er3-negative allele encodes wild-type Gly2394 (Era) with a nearby Glu2392Lys mutation. Exemplary mutations of PIEZO1 with resulting amino acid exchanges reported for patients with DHS are given in blue and are del756, Pro1358, Arg2225, Thr2020, and His2456. Exemplary mutations of PIEZO1 with resulting amino acid exchanges reported for patients with LMPHM6 are given in black and are Ter755, Ter1630, and Phe2171. All Er blood group antigens cluster in the carboxy-terminal loop of Piezo1. Dominant mutations causing DHS and LMPHM6 mutations following a recessive mode of inheritance may be observed throughout all parts of Piezo1. The position of the PIEZO1 mutations suggest coding of disease-associated alleles for blood group antigens and, vice versa, blood group antigens that may simultaneously represent disease-associated alleles. However, this hypothesis requires further research. Graphic generated in Protter.
Piezo1 protein (Uniprot Q92508) amino acids 602 to 2521 and known mutations in PIEZO1 encoding amino acid substitutions. Er blood group antigens encoded by PIEZO1 are given in red and as detailed by Karamatic Crew et al.1 Gly2394 is required for expression of the high-prevalence antigen Era, whereas Ser2394 encodes the antithetical low-prevalence antigen Erb. Proposed novel high-prevalence antigens Er4 and Er5 are associated, respectively, with Gln2407 and Arg2245 in Piezo1. One Er3-negative allele encodes wild-type Gly2394 (Era) with a nearby Glu2392Lys mutation. Exemplary mutations of PIEZO1 with resulting amino acid exchanges reported for patients with DHS are given in blue and are del756, Pro1358, Arg2225, Thr2020, and His2456. Exemplary mutations of PIEZO1 with resulting amino acid exchanges reported for patients with LMPHM6 are given in black and are Ter755, Ter1630, and Phe2171. All Er blood group antigens cluster in the carboxy-terminal loop of Piezo1. Dominant mutations causing DHS and LMPHM6 mutations following a recessive mode of inheritance may be observed throughout all parts of Piezo1. The position of the PIEZO1 mutations suggest coding of disease-associated alleles for blood group antigens and, vice versa, blood group antigens that may simultaneously represent disease-associated alleles. However, this hypothesis requires further research. Graphic generated in Protter.
Conflict-of-interest disclosure: C.G. acts as a consultant to inno-train Diagnostik, Kronberg im Taunus, Germany. Procedures for the molecular detection of GYPB deletions for S-s-U- phenotype diagnostics have been granted as a European patent (EP 3 545 102 B1). Similar-content US patent application is pending.