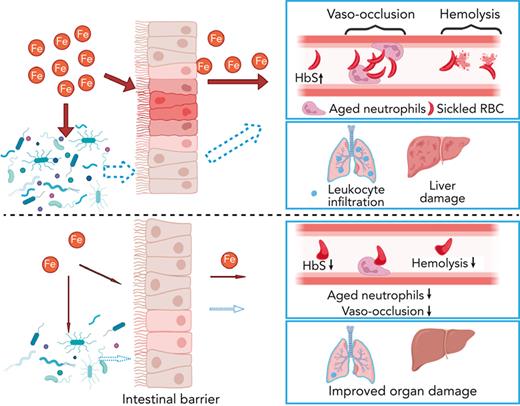
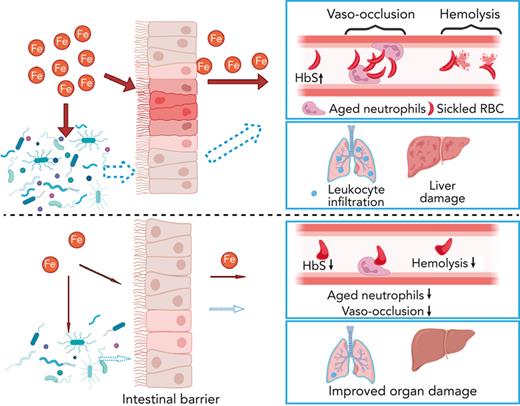
Key Points
Dietary iron restriction protects against both SCD vaso-occlusion and organ damage.
The benefits of dietary iron restriction in SCD are potentially mediated by regulation of gut microbiota-host crosstalk.
Abstract
Sickle cell disease (SCD) is an inherited disorder resulting from a β-globin gene mutation, and SCD patients experience erythrocyte sickling, vaso-occlusive episodes (VOE), and progressive organ damage. Chronic hemolysis, inflammation, and repeated red blood cell transfusions in SCD can disrupt iron homeostasis. Patients who receive multiple blood transfusions develop iron overload, and another subpopulation of SCD patients manifest iron deficiency. To elucidate connections between dietary iron, the microbiome, and SCD pathogenesis, we treated SCD mice with an iron-restricted diet (IRD). IRD treatment reduced iron availability and hemolysis, decreased acute VOE, and ameliorated chronic organ damage in SCD mice. Our results extend previous studies indicating that the gut microbiota regulate disease in SCD mice. IRD alters microbiota load and improves gut integrity, together preventing crosstalk between the gut microbiome and inflammatory factors such as aged neutrophils, dampening VOE, and organ damage. These findings provide strong evidence for the therapeutic potential of manipulating iron homeostasis and the gut microbiome to ameliorate SCD pathophysiology. Many treatments, which are under development, focus on lowering the systemic iron concentration to relieve disease complications, and our data suggest that iron-induced changes in microbiota load and gut integrity are related- and novel-therapeutic targets.
Introduction
Sickle cell disease (SCD) is the most commonly inherited hemoglobinopathy and results from a mutation in the β-globin gene (HbS), leading to red blood cell (RBC) sickling, vaso-occlusive episodes (VOE), and organ damage.1 Iron metabolism is dysregulated as a consequence of chronic hemolysis, inflammation, and recurrent RBC transfusion in SCD;2 and correlations between iron overload, organ damage development, and mortality have been reported.3 Iron deficiency is much less common, around 9% of individuals are affected,4 whereas 23% of patients develop iron overload secondary to repeated transfusions.3 Patient case reports highlight the role of iron restriction in reducing HbS concentration to improve clinical outcomes in SCD.5,6
We previously demonstrated that gut microbiota in SCD mice induce neutrophil aging, triggering a cascade of interactions that lead to heterocellular aggregates blocking vessels, thus initiating VOE.7 Gut microbiota are also implicated in organ damage where antibiotic depletion of microbiota alleviates splenomegaly and liver damage in SCD mice.7 Gut permeability is increased in both SCD patients and mice,8,9 potentially enabling increased translocation of microbial compounds, consequently triggering VOE and organ damage. Oral iron has been shown to worsen colitis in inflammatory bowel disease and is associated with changes in the gut microbiome.10 We sought to elucidate potential connections between dietary iron, microbiota, and SCD pathogenesis. Our study demonstrates that an iron-restricted diet (IRD) reduces VOE and mitigates organ damage in SCD mice. In addition to reducing HbS concentration which consequently reduces RBC sickling and hemolysis, beneficial outcomes may also potentially be mediated by the changes in gut microbiota-host crosstalk.
Study design
Intravital microscopy analysis of VOE
Inflamed cremasteric venules were imaged 2 hours after intrascrotal injection of 0.5 μg TNF-α using previously described methods.7
Organ damage analyses
Serum levels of alanine aminotransferase, aspartate aminotransferase, total bilirubin, and direct bilirubin were measured by the Biomarker-Analytic-Research Core at Albert Einstein College of Medicine.
Statistical analysis
Statistical significance between groups was determined by unpaired two-tailed t-tests using GraphPad-Prism-9 software.
Results and discussion
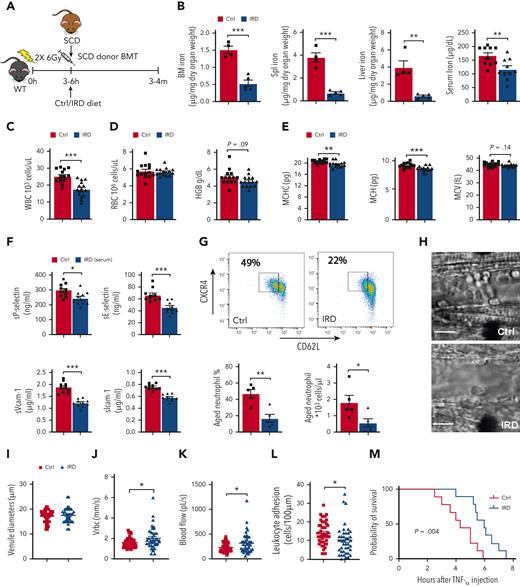
To study the impact of dietary iron on neutrophil aging and VOE initiation, we treated SCD mice with an IRD or a regular-iron diet (Ctrl) (Figure 1A). IRD reduced organ/serum iron concentration (Figure 1B) compared with Ctrl, confirming that IRD restricted iron availability in SCD mice. We confirmed that IRD did not delay bone-marrow engraftment and regeneration (supplemental Figure 1A-G,available on the Blood website). SCD patients with elevated white blood cells (WBCs) have an increased risk of developing stroke and acute chest syndrome, leading to premature death.11-13 In SCD mice, WBC counts were significantly lower in IRD than Ctrl group (Figure 1C & supplemental Figure 2A). No change in RBC counts and hemoglobin was observed suggesting anemia did not worsen in IRD compared with Ctrl group (Figure 1D). Reduced MCHC is associated with decreased RBC sickling and hemolysis in SCD.14,15 We demonstrated that IRD decreased MCHC and mean corpuscular hemoglobin (MCH) without impacting mean corpuscular volume (Figure 1E). Decreased MCHC implied reduced cellular HbS and correlated with lower serum indirect bilirubin suggesting a decrease in hemolysis in IRD (supplemental Figure 1B). Adhesion molecules (sP-selectin, sE-selectin, sVcam-1, and sIcam-1) were lower in IRD than Ctrl mice (Figure 1F). Furthermore, IRD reduced the fraction of aged neutrophils by >50% in SCD mice (Figure 1G), which corresponded with improved VOE parameters. Specifically, within equivalent diameter venules, intravital microscopy demonstrated elevated mean centerline RBC velocity and blood flow rate and decreased leukocyte adhesion in IRD than Ctrl (Figure 1H-L & supplemental Video 1). Rolling flux and WBC-RBC interaction rate were not different between the 2 groups (supplemental Figure 2C-D). Importantly, IRD prolonged survival after TNF-α challenge (Figure 1M) and protected SCD mice against VOE in response to TNF-α challenge.
Dietary ironrestriction amelioratesacute VOE in SCD mice. (A) Schematic experimental design for iron diet treatments. (B) Non-heme iron content of the bone marrow (N = 5), spleen (N = 5), and liver (N = 5), and serum iron concentration (N = 10) are decreased after dietary iron restriction. (C) WBC count, (D) RBC count, and total hemoglobin (HGB) concentration in Ctrl and IRD SCD mice (N = 14-15). (E) Mean corpuscular hemoglobin concentration (MCHC), MCH, mean corpuscular volume in Ctrl and IRD mice (merged data from 3 experiments, N = 14-15). (F) Serum soluble adhesion molecules (sP-selectin, sE-selectin, sVcam-1 and sIcam-1) by enzyme-linked immunosorbent assay (N = 10). (G) FACS plots with gating strategy and quantification of CXCR4Hi CD62Lo aged neutrophils in Ctrl and IRD mice. (H) Intravital microscopy images of inflamed cremasteric venules in Ctrl and IRD mice. Scale bars, 10 μm. (I) Cremasteric venule diameters, (J) red cell velocity (Vrbc), (K) blood flow rate, (L) the number of adherent leukocytes (N = 41-47), and (M) survival rate after TNF-α injection (N = 9) are compared between Ctrl and IRD mice. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
Dietary ironrestriction amelioratesacute VOE in SCD mice. (A) Schematic experimental design for iron diet treatments. (B) Non-heme iron content of the bone marrow (N = 5), spleen (N = 5), and liver (N = 5), and serum iron concentration (N = 10) are decreased after dietary iron restriction. (C) WBC count, (D) RBC count, and total hemoglobin (HGB) concentration in Ctrl and IRD SCD mice (N = 14-15). (E) Mean corpuscular hemoglobin concentration (MCHC), MCH, mean corpuscular volume in Ctrl and IRD mice (merged data from 3 experiments, N = 14-15). (F) Serum soluble adhesion molecules (sP-selectin, sE-selectin, sVcam-1 and sIcam-1) by enzyme-linked immunosorbent assay (N = 10). (G) FACS plots with gating strategy and quantification of CXCR4Hi CD62Lo aged neutrophils in Ctrl and IRD mice. (H) Intravital microscopy images of inflamed cremasteric venules in Ctrl and IRD mice. Scale bars, 10 μm. (I) Cremasteric venule diameters, (J) red cell velocity (Vrbc), (K) blood flow rate, (L) the number of adherent leukocytes (N = 41-47), and (M) survival rate after TNF-α injection (N = 9) are compared between Ctrl and IRD mice. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
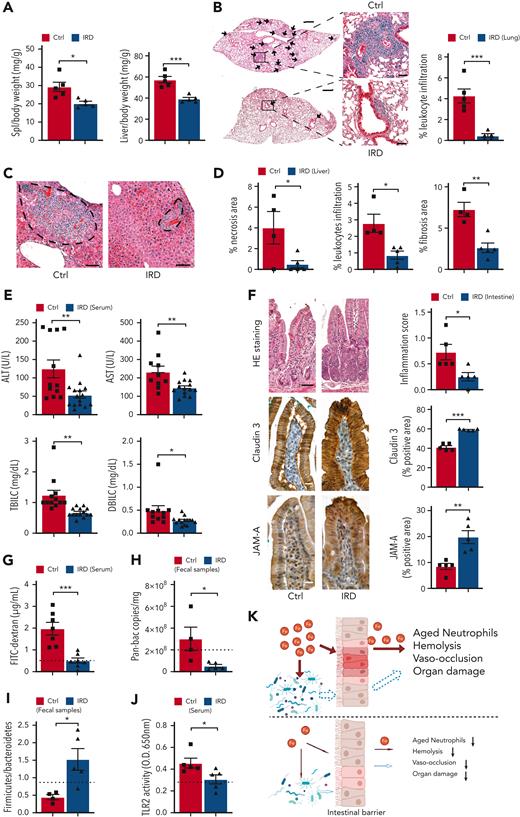
Next, we evaluated the effects of IRD on organ damage in SCD mice. IRD significantly reduced spleen and liver weight suggesting salutary effects on splenomegaly and hepatomegaly compared with Ctrl (Figure 2A). Inflammation is known to increase leukocyte infiltration into target organs.16,17 IRD resulted in a dramatic reduction in lung leukocyte infiltration, indicating significantly reduced lung inflammation in SCD mice (Figure 2B). There was significantly less liver necrosis, leukocyte infiltration and fibrosis (Figure 2C,D), decreased serum liver enzymes and reduced total and direct bilirubin levels in the IRD group than Ctrl group (Figure 2E). Overall, a reduced hepatocyte damage and improved liver function were seen in SCD in response to dietary iron restriction.
Organ damage parameters are improved in SCD mice after dietary iron restriction. (A) Gross spleen and liver weights are reduced in IRD group compared with Ctrl group. (N = 5) (B) Leukocyte infiltration in lung tissue, quantified by H&E staining, is decreased in IRD treated SCD mice. Low-magnification scale bar (left), 500 μm; high-magnification scale bar (right), 50 μm. (N = 5) (C) H&E staining of liver tissue; area within dashed line shows leukocyte infiltration and necrosis. Scale bar, 100 μm. (D) Liver necrosis, leukocyte infiltration, and fibrosis are all reduced in IRD mice. (N = 4-5) (E) Liver enzymes (alanine aminotransferase and aspartate aminotransferase), total bilirubin concentration, and direct bilirubin concentration from sera of Ctrl and IRD mice. (N = 10-15) (F) Left: representative images of duodenal villi with either H&E, anti-claudin 3 or anti-junctional adhesion molecule A (JAM-A) immunohistology staining in Ctrl and IRD treated SCD mice. H&E scale bar, 50 μm; immunohistology scale bar 20 μm. Right: quantification of intestinal inflammation or percentage of positive stained area. (N = 5) (G) Concentration of FITC-Dextran in serum of IRD mice is significantly lower than Ctrl mice. (N = 7) Dashed line represents levels in the healthy SA mice. (H) Bacterial load measured by Pan-bac quantitative reverse transcription polymerase chain reaction, in fecal samples normalized by the weight of fecal samples. (N = 4-5) Dashed line represents levels in the healthy SA mice. (I) Bacterial Firmicutes/Bacteroidetes ratio of fecal samples from Ctrl and IRD treated SCD mice. (N = 4-5) Dashed line represents levels in the healthy SA mice. (J) Quantification of sera TLR2 ligands activity in Ctrl and IRD treated SCD mice. (N = 5) Dashed line represents levels in the healthy SA mice. (K) Working model. Top: Excess dietary iron expands the proliferation of bacteria (red arrow indicates direct impact from iron) and increases gut permeability (red arrow) which allows the translocation of microbial compounds to internal organs (dashed arrow indicates indirect impact from iron); in parallel, excess iron is absorbed and deposited into organs (red arrow); together lead to exacerbation of neutrophil aging, hemolysis, vaso-occlusion, and organ damage. Bottom: dietary iron restriction reduces bacterial proliferation and ameliorates gut permeability, and consequently decreasing the translocation of microbial compounds and limiting systemic iron levels. Collectively, neutrophil aging, hemolysis, vaso-occlusion, and organ damage are found at lower rates. ∗P < .05, ∗∗P < .01, ∗∗∗P <.001.
Organ damage parameters are improved in SCD mice after dietary iron restriction. (A) Gross spleen and liver weights are reduced in IRD group compared with Ctrl group. (N = 5) (B) Leukocyte infiltration in lung tissue, quantified by H&E staining, is decreased in IRD treated SCD mice. Low-magnification scale bar (left), 500 μm; high-magnification scale bar (right), 50 μm. (N = 5) (C) H&E staining of liver tissue; area within dashed line shows leukocyte infiltration and necrosis. Scale bar, 100 μm. (D) Liver necrosis, leukocyte infiltration, and fibrosis are all reduced in IRD mice. (N = 4-5) (E) Liver enzymes (alanine aminotransferase and aspartate aminotransferase), total bilirubin concentration, and direct bilirubin concentration from sera of Ctrl and IRD mice. (N = 10-15) (F) Left: representative images of duodenal villi with either H&E, anti-claudin 3 or anti-junctional adhesion molecule A (JAM-A) immunohistology staining in Ctrl and IRD treated SCD mice. H&E scale bar, 50 μm; immunohistology scale bar 20 μm. Right: quantification of intestinal inflammation or percentage of positive stained area. (N = 5) (G) Concentration of FITC-Dextran in serum of IRD mice is significantly lower than Ctrl mice. (N = 7) Dashed line represents levels in the healthy SA mice. (H) Bacterial load measured by Pan-bac quantitative reverse transcription polymerase chain reaction, in fecal samples normalized by the weight of fecal samples. (N = 4-5) Dashed line represents levels in the healthy SA mice. (I) Bacterial Firmicutes/Bacteroidetes ratio of fecal samples from Ctrl and IRD treated SCD mice. (N = 4-5) Dashed line represents levels in the healthy SA mice. (J) Quantification of sera TLR2 ligands activity in Ctrl and IRD treated SCD mice. (N = 5) Dashed line represents levels in the healthy SA mice. (K) Working model. Top: Excess dietary iron expands the proliferation of bacteria (red arrow indicates direct impact from iron) and increases gut permeability (red arrow) which allows the translocation of microbial compounds to internal organs (dashed arrow indicates indirect impact from iron); in parallel, excess iron is absorbed and deposited into organs (red arrow); together lead to exacerbation of neutrophil aging, hemolysis, vaso-occlusion, and organ damage. Bottom: dietary iron restriction reduces bacterial proliferation and ameliorates gut permeability, and consequently decreasing the translocation of microbial compounds and limiting systemic iron levels. Collectively, neutrophil aging, hemolysis, vaso-occlusion, and organ damage are found at lower rates. ∗P < .05, ∗∗P < .01, ∗∗∗P <.001.
We further hypothesized that, in addition to reducing organ iron concentration, IRD ameliorates organ damage by preventing translocation of microbial compounds. IRD led to reduced intestinal inflammation (Figure 2F) and restored gut permeability by strengthening tight junctions, indicated by elevated expression of Claudin-3 and junctional adhesion molecule A compared with Ctrl (Figure 2F). Increased junctional adhesion molecule A has been reported to decrease intestinal inflammation and leukocyte infiltration,18 which may explain the reduced intestinal inflammation observed in IRD (Figure 2F). IRD inhibited translocation of FITC-Dextran by 5-fold compared with Ctrl, suggesting functionally improved gut permeability (Figure 2G). IRD also decreased fecal bacterial load in SCD mice (Figure 2H). Firmicutes/Bacteroidetes ratio is low in SCD patients and mice suggesting dysbiosis.19 In IRD SCD mice, Firmicutes/Bacteroidetes ratio was increased to the level similar to healthy heterozygous SA mice (Figure 2I). Using a murine toll-like receptor 2 (TLR2) reporter cell line, we detected significantly decreased TLR2 ligands in serum of IRD mice (Figure 2J). Serum lipopolysaccharide levels were not detectable by enzyme-linked immunosorbent assay (data not shown) suggesting ligands from gram-positive bacteria (TLR2 agonists) may play a greater role in SCD complications than lipopolysaccharides. In our working model, IRD collectively lowers iron availability, improves hemolysis, dampens activation of aged neutrophils, decreases gut bacteria, strengthens intestinal barriers, and reduces translocation of microbial compounds such as TLR2 ligands into the circulation, ameliorating VOE and organ damage in SCD mice (Figure 2K).
Our study demonstrates for the first time that dietary iron restriction reduces VOE severity and alleviates chronic organ damage in SCD mice. In Townes SCD mice, knocking-out intestine iron absorption protein HIF-2α20 by 6 months of age or restricting dietary iron intake at weaning age14 increases total hemoglobin concentration, prevents RBC sickling, and suppresses hemolysis. Administration of ferroportin inhibitor in Townes SCD mice aged 1 to 2 months restricts iron availability, and lowers RBC sickling and hemolysis.21 In our bone-marrow transplanted Berkeley SCD mice on IRD, we consistently observed reduction in cellular HbS concentration and hemolysis but without changes in total hemoglobin after 4 months of IRD treatment. Although organ iron deposition is significantly reduced in HIF-2α–depleted SCD mice, no changes in spleen and liver manifestations were observed,20 suggesting that intestinal luminal iron is implicated in SCD organ damage. Dietary iron is known to expand pathogenic bacteria and may promote intestinal inflammation.22,23 We speculate that the regulation of microbiota-host crosstalk by IRD contribute to improvement of VOE and organ damage in SCD mice.
In addition to iron-chelators, the regulation of iron availability using hepcidin-mimetics and ferroportin inhibitors may provide significant benefits in relieving iron overload in SCD.21,24,25 Nevertheless, dietary iron restriction is a viable therapeutic strategy in iron-overloaded SCD patients and has the potential to modify the disease further. Although iron restriction would not be desirable in the smaller subset of SCD patients that are iron deficient, substitution of parenteral iron administration and avoidance of oral iron are important considerations. Therapies that directly target the gut microbiome-aged neutrophil crosstalk and gut barrier integrity provide a novel area for further investigation with the potential of improving hemolysis, VOE, and organ damage in SCD patients.
Acknowledgments
The authors thank Colette Prophete and Peng Guo for technical assistance and members of the Frenette laboratory for helpful discussions.
This work was supported by the R01 grant from the National Heart, Lung, and Blood Institute (R01 HL069438 to P.S.F and L.K.). H.L. acknowledges the support of the National Heart, Lung, and Blood Institute (1F32HL142243) and National Institute of Diabetes and Digestive and Kidney Diseases (1K01DK131401). Y.Z.G. acknowledges the support of the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK107670 and DK095112). M.M. was supported by the EMBO European Commission FP7 (Marie Curie Actions; EMBOCOFUND2012, GA-2012-600394, ALTF 447-2014), by the New York Stem Cell Foundation Druckenmiller fellowship, and by the American Society of Hematology Research Restart Award. We thank the Flow Cytometry, Histopathology, and Analytical Imaging of Albert Einstein College of Medicine for technical assistance, supported by P250 high-capacity slide scanner (1S10OD019961-01).
Authorship
Contribution: H.L. designed and performed experiments, analyzed data and wrote the manuscript; J.S.K. participated in experiments, data analysis, and the manuscript preparation; S.L., D.Z., X.G., M. M., L.T., and D.V. advised on experimental design and helped with experiments; L.K. and Y.Z.G. participated in study design and revised the manuscript; P.S.F. supervised the study; D.M. supervised the study and wrote the manuscript; and all authors discussed the results and contributed to the manuscript.
Conflict-of-interest disclosure: The authors declare no relevant competing financial interests.
Correspondence: Huihui Li, Albert Einstein College of Medicine, Department of Systems and Computational Biology, Department of Cell Biology, 1301 Morris Park Ave, Price center, Room 107, Bronx, NY, 10461; e-mail: huihui.li@einsteinmed.edu; and Deepa Manwani, Albert Einstein College of Medicine, Division of Pediatric Hematology/Oncology, Department of Pediatrics, 3411 Wayne Ave, Bronx, NY 10467; e-mail: dmanwani@montefiore.org.
References
Author notes
Data are available upon reasonable request from the corresponding authors, Huihui Li (huihui.li@einsteinmed.edu) and Deepa Manwani (dmanwani@montefiore.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.