Key Points
Intracellular RBC polymorphisms can facilitate alloimmunization against completely unrelated RBC surface antigens.
Immune responses to undetected intracellular polymorphisms may explain higher alloimmunization rates among some transfusion recipients.
Abstract
Antibodies against red blood cell (RBC) alloantigens can increase morbidity and mortality among transfusion recipients. However, alloimmunization rates can vary dramatically, as some patients never generate alloantibodies after transfusion, whereas others not only become alloimmunized but may also be prone to generating additional alloantibodies after subsequent transfusion. Previous studies suggested that CD4 T–cell responses that drive alloantibody formation recognize the same alloantigen engaged by B cells. However, because RBCs express numerous antigens, both internally and externally, it is possible that CD4 T–cell responses directed against intracellular antigens may facilitate subsequent alloimmunization against a surface RBC antigen. Here, we show that B cells can acquire intracellular antigens from RBCs. Using a mouse model of donor RBCs expressing 2 distinct alloantigens, we demonstrate that immune priming to an intracellular antigen, which would not be detected by any currently used RBC compatibility assays, can directly influence alloantibody formation after exposure to a subsequent distinct surface RBC alloantigen. These findings suggest a previously underappreciated mechanism whereby transfusion recipient responders may exhibit an increased rate of alloimmunization because of prior immune priming toward intracellular antigens.
Introduction
Exposure to red blood cell (RBC) alloantigens can result in alloantibody formation, which can delay procurement of compatible RBCs and can increase rates of adverse transfusion reactions.1-11 Although not all individuals receiving transfusion develop alloantibodies after RBC transfusion, some patients, referred to as responders, appear to be particularly susceptible to alloantibody formation.1,6,12-16 Even among responders, some are particularly prone to generating additional alloantibodies after subsequent transfusion and are referred to as hyperresponders.12,17,18 However, the reason some individuals are more susceptible to RBC alloimmunization remains unclear. Several studies suggest that differences in immune function and recipient inflammation may predispose individuals to become alloimmunized.19-28 Although these factors likely influence RBC alloimmunization, whether other immunological priming events similarly enhance alloantibody formation remains incompletely defined.
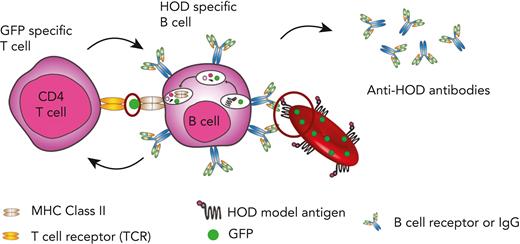
Previous studies on RBC alloimmunization and adaptive immunity, in general, suggest that CD4 T cells primarily enhance B-cell responses after recognition of peptides that are directly part of, or at least linked to, the antigen recognized by the cognate B-cell receptor.29,30 This help provided by CD4 T cells has been shown to be important as an additional safeguard in the establishment of tolerance and forms the basis of conjugate vaccine design as a fundamental principle in immunology.31-34 However, because RBCs express a variety of polymorphisms inside and outside the cell, preexisting immunity against a polymorphic alloantigen not directly bound to a target surface alloantigen may influence RBC alloimmunization. Consistent with this, alloimmunization against a surface alloantigen may enhance subsequent alloantibody formation against a distinct surface alloantigen after subsequent transfusion.35 Although this process suggests that membrane fragments that contain other surface alloantigens, in addition to the specific alloantigen, targets themselves may be taken up by B cells to facilitate alloimmunization; the ability of some patients to respond to a wide variety of distinct RBC alloantigens, even when transfused RBCs are matched for alloantigens against which the patient is currently alloimmunized, suggests that alloantigens not routinely detected clinically could prime future RBC alloimmunization.36 Among possible antigens in this scenario, intracellular alloantigens may play a significant role. However, as prior examples of trogocytosis, the process in which cells capture portions of other cells without compromising the viability of either of the cells, have been predominantly evaluated for functional consequence of membrane receptor translocation;37,38 whether this process can similarly result in removal of cytoplasmic contents that could facilitate the development of an immune response remains untested. These collective data suggest that in addition to the underlying differences in immune function, prior priming events could sensitize an individual against alloantigens not detected clinically and in turn enhance the probability of RBC alloimmunization. However, the formal role of cytosolic antigens in driving alloimmunization remains unexplored.
Deliberate exposure to RBC alloantigens with the purpose of studying the development of alloantibodies is clinically unethical, because only ABO (H) and RhD are matched in the emergent setting,39-41 so we turned to using a murine model of RBC alloimmunization to define the possible impact of prior priming toward an intracellular antigen on subsequent alloimmunization. To accomplish this, we crossed the ubiquitin (Ub) green fluorescent protein (GFP) mice, which express GFP intracellularly under the control of a Ub promoter, with either hen-egg lysozyme (HEL) fused with ovalbumin and human Duffy (HOD) or glycophorin A (GPA) transgenic mice.42-44 Using this approach, our results demonstrate that prior priming with GFP sensitizes recipients to generate an enhanced response to HOD or GPA after transfusion of RBCs that express either both HOD and GFP (HOD × GFP) or GPA and GFP (GPA × GFP). Taken together, these results suggest that prior priming events toward an intracellular antigen may directly enhance RBC alloimmunization, providing a possible mechanism that could increase alloimmunization after transfusion.
Methods
Mice
Wild-type (WT) C57BL/6 (B6) mice were purchased from Charles River. IgheIMD4 transgenic (MD4) mice, which produce B cells specific to the HEL antigen and Ub-B6.GFP transgenic mice, which expressed soluble GFP intracellularly under a Ub promoter, were purchased from the Jackson Laboratory.42 HOD and GPA mice were generated as previously described.43,44 HOD × GFP F1 and GPA × GFP F1 donors were generated by breeding HOD with GFP mice and GPA with GFP mice, respectively. Experimental protocols and animal procedures were approved by the Emory University or the Brigham and Women’s Hospital institutional animal care and use committees.
GFP protein expression, purification, and coupling to B6 RBCs
GFP cloned into a pGEX-4T1 vector was used to transform Escherichia coli BL21 (DE3), which was cultured in Luria-Bertani broth containing 100 μg/mL ampicillin at 37°C until the midlog phase (optical density, 0.4-0.6). Protein expression was induced using isopropyl 1-thio-β-d-galactopyranoside (IPTG; 1 mM). After 20-hour induction at 16°C, bacteria were pelleted and lysed, and the released GFP was purified over a glutathione Sepharose 4B resin (GE17-0756-01), per manufacturer’s instructions. Recombinant GFP was derivatized from N-hydroxysulfosuccinimide (Thermo Fisher Scientific) in accordance with the manufacturer’s protocol and was then coupled with RBCs, as outlined previously.45
Evaluation of HOD × GFP and GPA × GFP RBCs
For flow cytometry analysis, WT (C57BL/6), HOD, GPA, GFP, HOD × GFP, and GPA × GFP RBCs were stained with anti-Duffy, anti-HEL, anti-GFP, or anti-GPA, as indicated, followed by detection using anti-mouse immunoglobulin G (IgG), as carried out previously.46-52 For immunofluorescent superresolution microscopy, 1 mL of 0.001% poly-L-lysine (Sigma) in phosphate-buffered saline (PBS) was added to each 15 mm glass coverslip and incubated for 1 hour. Each coverslip was then washed thrice with PBS and air-dried. RBCs were stained as described earlier, then added to coverslips and incubated for 30 minutes on ice. Cells were fixed in 1% paraformaldehyde, followed by imaging using a Nikon N-SIM superresolution microscope.
GFP internalization
Fifty μl packed HOD, GFP, or HOD × GFP RBCs in a total volume of 300 μl RBCs were transfused into MD4 recipients (for recipients that received HOD × GFP RBCs, 50 μl of each type was transfused). Splenocytes were harvested at 4 hour after transfusion, washed thrice in cold PBS with 2% bovine serum albumin and then incubated with Zombie Yellow viability stain, B220, CD3, and Ter119 antibodies (1:100, BioLegend, San Diego, CA) for 30 minutes on ice.
GFP immunization, passive immunization, RBC transfusion, tetramer staining, and seroanalysis
B6 recipients were primed using the same approach outlined previously, by administering intraperitoneal injections of ∼10 × 106 total GFP splenocytes at 1-week intervals for 3 weeks;53 control recipients received B6 splenocytes parallelly. B6 mice were passively immunized with 50 μl GFP-primed serum or 100 μg anti-Duffy antibody. Passively immunized GFP or B6 primed or nonprimed recipients were transfused with 50 μl of each RBC population (for recipients receiving HOD × GFP RBCs or GPA × GFP RBCs, 50 μl of each type was transfused) in a total volume of 300 μl PBS, as outlined previously.46-52 Serum or whole blood was collected at the time points indicated, followed by analyzing for antibody formation via flow cross match, as previously described,46-52 or for RBC survival, IgG binding, HOD antigen, or TER119 levels as previously described.52,54 HEL tetramers and overall analyses were accomplished as outlined previously.50 Flow cytometry was accomplished using a BD FACSCalibur, a BD LSR II, or a Cytek Northern Lights instrument. FlowJo software was used to analyze each data set. For anti-GFP antibody detection plates were coated with 0.5 μg/mL GFP, blocked with 1% bovine serum albumin, and probed with a 1:400 dilution of serum for 2 hours, followed by detection of anti-mouse IgM horseradish peroxidase or anti-mouse IgG horseradish peroxidase, as outlined previously.55-57
Statistical analysis
GraphPad Prism 8.4.2 software was used for statistical analysis. Two groups were analyzed using t tests, whereas ≥3 groups were analyzed using a 1-way analysis of variance along with Tukey multiple comparison test. P ≤ .05 was defined as statistically significant.
Results
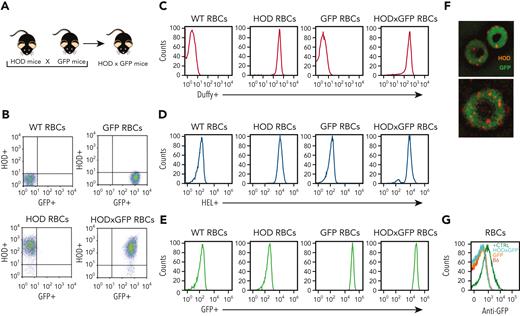
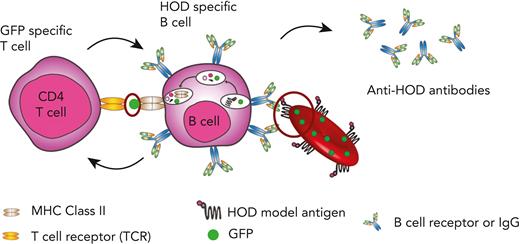
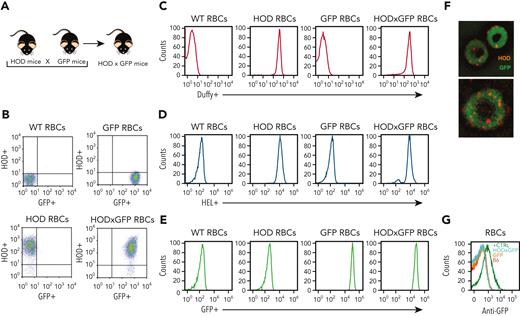
Although B cells are antigen presenting cells, they are not considered capable of engulfing a whole RBC24,58-66 However, the ability of some patients to respond to multiple RBC alloantigens suggests that prior immunological memory may crossboost antibody formation against unrelated RBC antigens.63 These results suggest that alloantigens not detected using routine serological assays may be involved in RBC alloimmunization. B-cell internalization of intracellular RBC contents, including possible intracellular antigens, may facilitate this process. To test this, we crossed HOD and Ub-GFP mice to generate HOD × GFP mice that produce RBCs expressing HOD and GFP (HOD × GFP RBCs) (Figure 1A), with GFP being a completely unrelated intracellular protein not known to specifically interact with any RBC alloantigen. To verify the expression and localization of HOD and GFP antigens, RBCs from HOD, GFP, and HOD × GFP donors were stained with anti-HEL, anti-Duffy, and anti-GFP antibodies. Although both the HEL and Duffy components of the HOD antigen could be detected on the surface of HOD and HOD × GFP RBCs, these antigens were not detected on GFP RBCs surface (Figure 1B-E). Consistent with the observation with regard to the intracellular localization of GFP, no GFP expression could be observed on the cell surface of HOD, GFP, or HOD × GFP RBCs but could be detected intracellularly in both GFP and HOD × GFP RBCs via confocal analysis (Figure 1F). However, to ensure that failure of anti-GFP antibody to detect GFP was not a consequence of its inability to engage GFP when present on the RBC surface, we had the GFP protein recombinantly expressed in the mice and chemically coupled this antigen to the RBC surface. Although the anti-GFP was not able to detect GFP on the cell surface of GFP or HOD × GFP RBCs, GFP was readily detectable on the surface of control RBCs in which GFP had been chemically coupled to the RBC surface (Figure 1G), strongly suggesting that GFP is likely confined to the intracellular compartment in GFP and HOD × GFP RBCs.
Characterization of HOD × GFP RBCs. (A) Schematic of mouse breeding used to produce HOD × GFP mice. (B-C) Flow cytometric analysis displayed as dot plots (B) or histograms (C) of WT RBCs, HOD RBCs, GFP RBCs, and HOD × GFP RBCs after staining with anti-Duffy antibody. (D) Flow cytometric analysis of WT RBCs, HOD RBCs, GFP RBCs, and HOD × GFP RBCs after staining with anti-HEL antibody. (E) Detection of endogenous GFP expression by flow cytometric analysis of WT RBCs, HOD RBCs, GFP RBCs, and HOD × GFP RBCs. (F) Representative images of HOD × GFP RBCs. (G) Flow cytometric analysis of WT (B6) RBCs, GFP RBCs, HOD × GFP RBCs, and WT RBCs with chemically coupled recombinant GFP (positive control) after staining with anti-GFP antibody. All panels show representative data from experiments reproduced 2 times.
Characterization of HOD × GFP RBCs. (A) Schematic of mouse breeding used to produce HOD × GFP mice. (B-C) Flow cytometric analysis displayed as dot plots (B) or histograms (C) of WT RBCs, HOD RBCs, GFP RBCs, and HOD × GFP RBCs after staining with anti-Duffy antibody. (D) Flow cytometric analysis of WT RBCs, HOD RBCs, GFP RBCs, and HOD × GFP RBCs after staining with anti-HEL antibody. (E) Detection of endogenous GFP expression by flow cytometric analysis of WT RBCs, HOD RBCs, GFP RBCs, and HOD × GFP RBCs. (F) Representative images of HOD × GFP RBCs. (G) Flow cytometric analysis of WT (B6) RBCs, GFP RBCs, HOD × GFP RBCs, and WT RBCs with chemically coupled recombinant GFP (positive control) after staining with anti-GFP antibody. All panels show representative data from experiments reproduced 2 times.
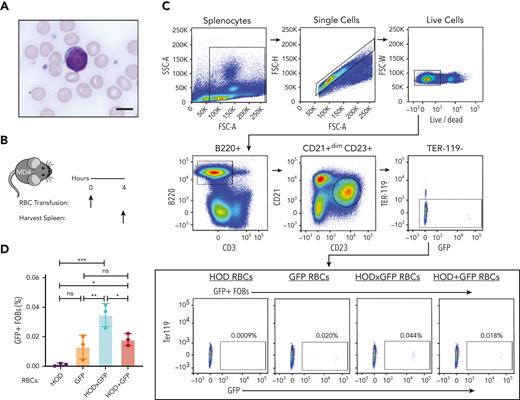
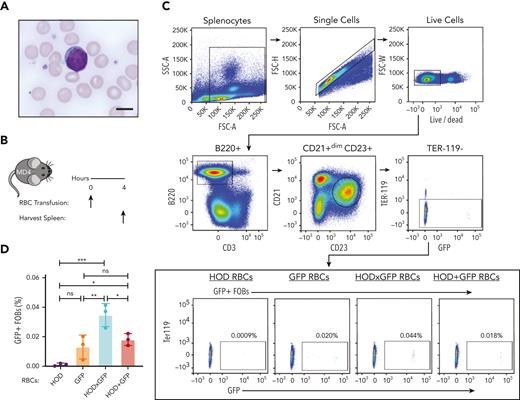
Despite the B cells from MD4 transgenic mice, which possessed B cells that expressed a B-cell receptor (BCR) for HEL (the component of HOD responsible for B-cell response), displaying a scant cytoplasmic rim when examined in the presence of RBCs (Figure 2A), we next sought to determine whether B cells specific to HOD can internalize GFP after HOD × GFP RBCs transfusion. To accomplish this, we transfused MD4 transgenic mice with HOD, GFP, HOD × GFP, or a mixture of HOD and GFP RBCs (HOD + GFP) (Figure 2B). Absence of RBC surface marker Ter119 detection was used to selectively assess GFP internalization by B cells after the transfusion of different RBC groups (Figure 2C). MD4 recipients demonstrated a statistically significant increase in GFP positivity after HOD × GFP RBC transfusion when compared with HOD, GFP, or HOD + GFP RBCs transfusion (Figure 2C and D).
HOD-reactive MD4 B cells endocytose the intracellular antigen GFP. (A) Blood smear image showing mouse RBCs and a MD4 B cell. Scale bar, 6 μm. (B) Experimental schematic of MD4 recipients transfused with HOD RBCs, GFP RBCs, HOD × GFP RBCs, or HOD + GFP RBCs. (C) Flow cytometric gating strategy for detecting GFP + MD4 B cells. (D) Percent B-cell endocytosis of GFP after transfusion of each RBC population is shown. Error bars represent mean ± standard deviation (SD). All panels show representative data from experiments reproduced 2 times. ∗ P ≤ .05, ∗∗ P ≤ .01, ∗∗∗ P ≤ .001. FSC, forward scatter; ns, not significant; SSC, side scatter.
HOD-reactive MD4 B cells endocytose the intracellular antigen GFP. (A) Blood smear image showing mouse RBCs and a MD4 B cell. Scale bar, 6 μm. (B) Experimental schematic of MD4 recipients transfused with HOD RBCs, GFP RBCs, HOD × GFP RBCs, or HOD + GFP RBCs. (C) Flow cytometric gating strategy for detecting GFP + MD4 B cells. (D) Percent B-cell endocytosis of GFP after transfusion of each RBC population is shown. Error bars represent mean ± standard deviation (SD). All panels show representative data from experiments reproduced 2 times. ∗ P ≤ .05, ∗∗ P ≤ .01, ∗∗∗ P ≤ .001. FSC, forward scatter; ns, not significant; SSC, side scatter.
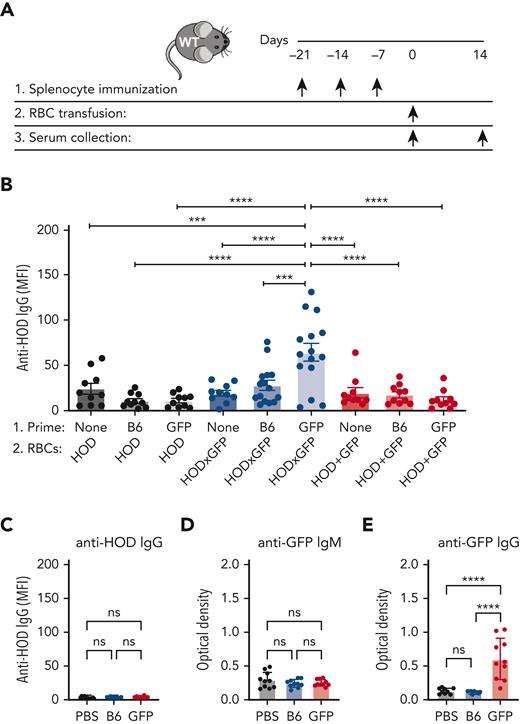
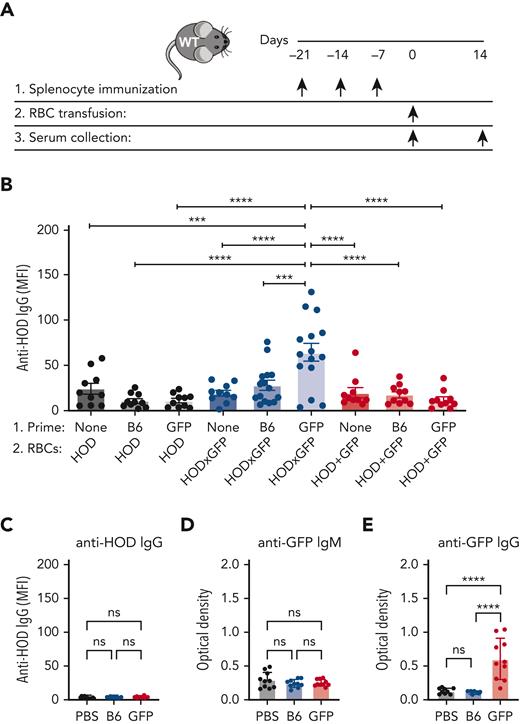
Given the ability of HOD-specific B cells to internalize GFP after HOD × GFP RBC transfusion, we next sought to determine whether prior priming with GFP may affect alloantibody formation against HOD after subsequent HOD × GFP RBC transfusion. To accomplish this, we first primed B6 recipients with GFP, followed by transfusion with HOD, HOD × GFP, or HOD + GFP RBCs (Figure 3A). Although transfusion of HOD × GFP RBCs produced a marginal alloantibody response, prior priming with GFP resulted in a significant anti-HOD antibody boost after HOD × GFP RBC transfusion (Figure 3B). The ability of GFP priming to boost HOD required GFP expression in HOD RBCs because similar transfusions of HOD + GFP RBCs or HOD RBCs alone did not result in the same boosting effect (Figure 3B); this was consistent with the notion that GFP must be internalized by HOD-reactive B cells to receive help in primed recipients.
Priming to intracellular GFP facilitates antibody formation against the surface HOD antigen after HOD × GFP RBC exposure. (A) Experimental schematic of GFP priming, transfusion, and serum collection. (B) Anti-HOD IgG formation after HOD, HOD × GFP, or HOD + GFP RBC transfusion. (C) Anti-HOD IgG formation examined in PBS-treated, GFP-primed or B6-exposed recipients. (D-E) Anti-GFP IgM (D) or IgG (E) formation examined in PBS-treated, GFP-primed or B6-exposed recipients. Error bars represent mean ± SD. All panels show combined data from experiments reproduced at least 2 times. ∗∗∗ P ≤ .001 and ∗∗∗∗ P ≤ .0001. ns, not significant.
Priming to intracellular GFP facilitates antibody formation against the surface HOD antigen after HOD × GFP RBC exposure. (A) Experimental schematic of GFP priming, transfusion, and serum collection. (B) Anti-HOD IgG formation after HOD, HOD × GFP, or HOD + GFP RBC transfusion. (C) Anti-HOD IgG formation examined in PBS-treated, GFP-primed or B6-exposed recipients. (D-E) Anti-GFP IgM (D) or IgG (E) formation examined in PBS-treated, GFP-primed or B6-exposed recipients. Error bars represent mean ± SD. All panels show combined data from experiments reproduced at least 2 times. ∗∗∗ P ≤ .001 and ∗∗∗∗ P ≤ .0001. ns, not significant.
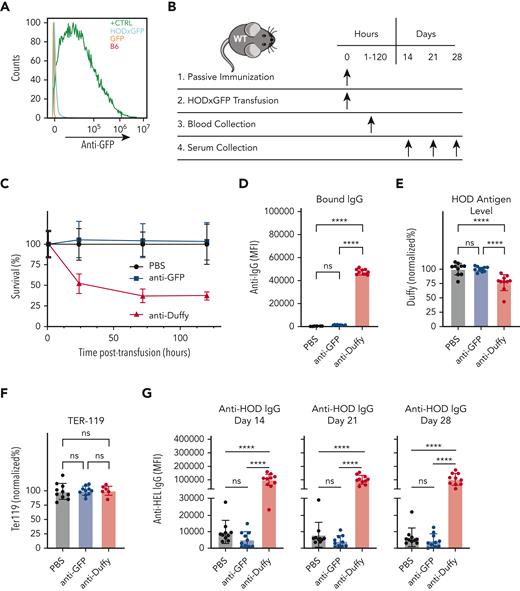
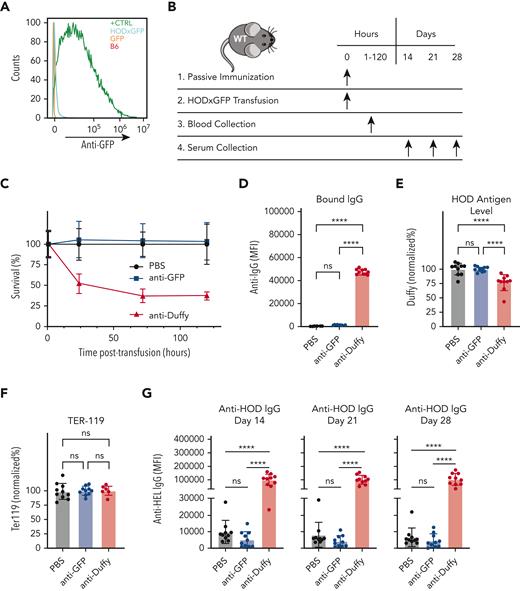
Within Ub-GFP mice, GFP is expressed internally and therefore is not accessible to surface binding with BCRs. However, it remains possible that after initial priming events, GFP becomes exposed to the extracellular space, allowing a humoral GFP immune response to develop. This raises the possibility that anti-GFP antibodies may drive enhanced anti-HOD antibody formation after HOD × GFP RBC transfusion. This outcome would be intriguing when considering that antibodies directed toward intracellular antigens are not examined clinically. To explore this, we first examined whether GFP priming results in anti-GFP antibody formation. Consistent with this possibility, although anti-HOD antibodies were not present in GFP-primed recipients, anti-GFP antibodies were present in the serum after GFP priming (Figure 3C-E). To determine whether anti-GFP antibodies play a role in the observed anti-HOD antibody boost, we assessed whether anti-GFP antibodies possess the ability to engage HOD × GFP RBCs. Not only did the serum from GFP-primed recipients fail to bind with HOD × GFP RBCs, but the transfusion of HOD × GFP RBCs into anti-GFP immunized recipients also failed to result in any detectable change with regard to antibody deposition, HOD antigen levels, or HOD × GFP RBC survival (Figure 4A-F). In contrast, the parallel transfusion of HOD × GFP RBCs into anti-Duffy passively immunized recipients, as a control procedure, resulted in significant antibody deposition, reduced HOD antigen levels, and accelerated HOD × GFP removal (Figure 4A-F), consistent with previously obtained results.67,68 These results mirror the lack of anti-GFP binding observed in vitro and strongly suggest that anti-GFP antibodies do not engage or affect the survival of HOD × GFP RBCs in vivo.
Anti-GFP antibody does not mediate enhancement of anti-HOD antibody formation. (A) Flow cytometric analysis of B6 RBCs, GFP RBCs, HOD × GFP RBCs, and B6 RBCs chemically coupled with recombinant GFP after staining with serum from GFP primed recipients. (B) Experimental schematic of passive immunization and evaluation of antibody deposition, antigen levels, RBC clearance, and alloimmunization. (C) Percent survival of HOD × GFP RBCs after transfusion into nonimmunized controls or into recipients who were passively immunized with anti-GFP antibodies or anti-Duffy. (D-F) Assessment of IgG antibody binding (D), detection of RBC surface HOD (E), or detection of RBC surface Ter119 (F) one hour after transfusion of HOD × GFP RBCs into nonimmunized, anti-GFP antibody, or anti-Duffy immunized recipients. (G) Anti-HOD IgG detected at 14, 21, or 28 days after transfusion into nonimmunized passively immunized recipient as indicated. Error bars represent mean ± SD. ∗∗∗∗ P ≤ .0001. ns, not significant.
Anti-GFP antibody does not mediate enhancement of anti-HOD antibody formation. (A) Flow cytometric analysis of B6 RBCs, GFP RBCs, HOD × GFP RBCs, and B6 RBCs chemically coupled with recombinant GFP after staining with serum from GFP primed recipients. (B) Experimental schematic of passive immunization and evaluation of antibody deposition, antigen levels, RBC clearance, and alloimmunization. (C) Percent survival of HOD × GFP RBCs after transfusion into nonimmunized controls or into recipients who were passively immunized with anti-GFP antibodies or anti-Duffy. (D-F) Assessment of IgG antibody binding (D), detection of RBC surface HOD (E), or detection of RBC surface Ter119 (F) one hour after transfusion of HOD × GFP RBCs into nonimmunized, anti-GFP antibody, or anti-Duffy immunized recipients. (G) Anti-HOD IgG detected at 14, 21, or 28 days after transfusion into nonimmunized passively immunized recipient as indicated. Error bars represent mean ± SD. ∗∗∗∗ P ≤ .0001. ns, not significant.
Although no obvious changes in RBC clearance or antibody deposition were observable in recipients passively immunized with anti-GFP antibodies, it remained possible that anti-GFP antibodies may still influence alloimmune responses after HOD × GFP RBC transfusion, either because of possible interactions not detectable via flow cytometry or through a mechanism entirely independent of HOD × GFP RBC engagement. To directly assess whether anti-GFP antibodies possess the capacity to alter anti-HOD antibody formation after HOD × GFP RBC transfusion, recipients were passively immunized with anti-GFP antibodies, followed by HOD × GFP RBC transfusion; for the control group, recipients were, in parallel, passively immunized with anti-Duffy. Although recipients passively immunized with anti-Duffy antibody made significantly more anti-HOD antibodies than control recipients, passive immunization with anti-GFP antibodies failed to enhance anti-HOD antibody production (Figure 4G). Together, these data suggest that although anti-GFP antibodies are produced after GFP priming, these antibodies do not appear to possess the capacity to enhance the anti-HOD antibody response after HOD × GFP RBC transfusion.
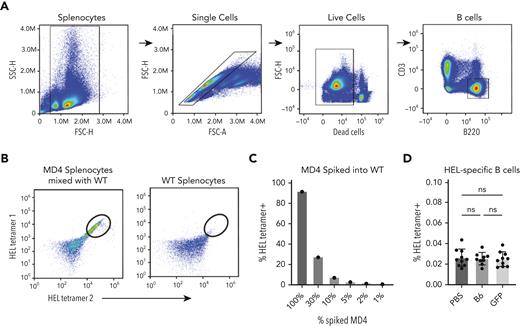
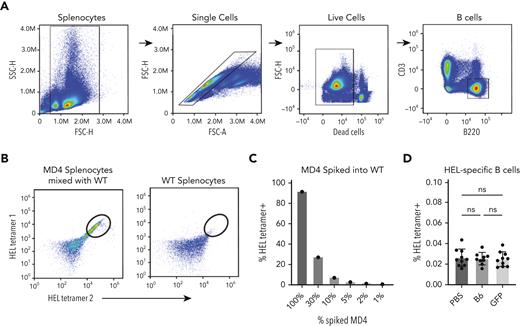
Because anti-GFP antibodies did not appear to enhance anti-HOD alloimmunization, we next sought to define the possible impact of GFP priming directly on HEL-specific B cells; possible GFP-induced enhancement of HEL-specific B-cell numbers could reflect an off-target effect of GFP priming that could account for the enhanced response observed after HOD × GFP RBC transfusion. To test this, we generated HEL-specific tetramers through biotinylation of HEL and captured with fluorescently labeled streptavidin to detect HEL-specific B cells. Assessment of antigen-specific B cells via tetramer staining demonstrated that HEL-specific B cells could be readily detected in MD4 mice, in which the number of HEL-specific B cells detected were proportional to the MD4 B-cell input, demonstrating the feasibility of this approach (Figure 5A-C). To determine whether GFP priming changes HEL-specific B-cell numbers, we examined HEL tetramer–positive B cells from GFP-primed and control recipients. Although HEL-specific B cells were detectable in each recipient, the number of HEL-specific B cells did not differ between these groups (Figure 5D), strongly suggesting that GFP priming does not affect HEL-specific B-cell numbers. Taken together, these combined results suggest that GFP priming does not enhance subsequent anti-HOD antibody formation through alterations induced by anti-GFP antibodies or through an expansion of HEL-specific B cells that in turn increase the immune response to HOD after HOD × GFP RBC transfusion.
Priming with GFP does not increase HEL-specific B-cell numbers. (A) Gating strategy for detection of total B cells via flow cytometry. (B) Gating strategy for detection of HEL-specific B cells using dual tetramer staining. Example dot plots show results after incubation of HEL-specific tetramers with a mixture of WT splenocytes and splenocytes from HEL-specific B-cell transgenic MD4 mice or WT splenocytes alone. (C) Quantification of percent HEL tetramer detection of HEL-specific MD4 B cells after mixing with WT splenocytes at defined ratios. (D) Quantification of percent HEL tetramer–positive B cells examined in PBS-treated, GFP-primed or B6-exposed recipients. Error bars represent mean ± SD. FSC, forward scatter; ns, no significance; SSC, side scatter.
Priming with GFP does not increase HEL-specific B-cell numbers. (A) Gating strategy for detection of total B cells via flow cytometry. (B) Gating strategy for detection of HEL-specific B cells using dual tetramer staining. Example dot plots show results after incubation of HEL-specific tetramers with a mixture of WT splenocytes and splenocytes from HEL-specific B-cell transgenic MD4 mice or WT splenocytes alone. (C) Quantification of percent HEL tetramer detection of HEL-specific MD4 B cells after mixing with WT splenocytes at defined ratios. (D) Quantification of percent HEL tetramer–positive B cells examined in PBS-treated, GFP-primed or B6-exposed recipients. Error bars represent mean ± SD. FSC, forward scatter; ns, no significance; SSC, side scatter.
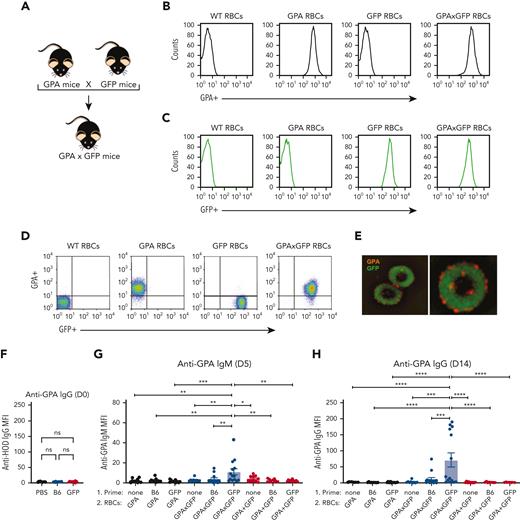
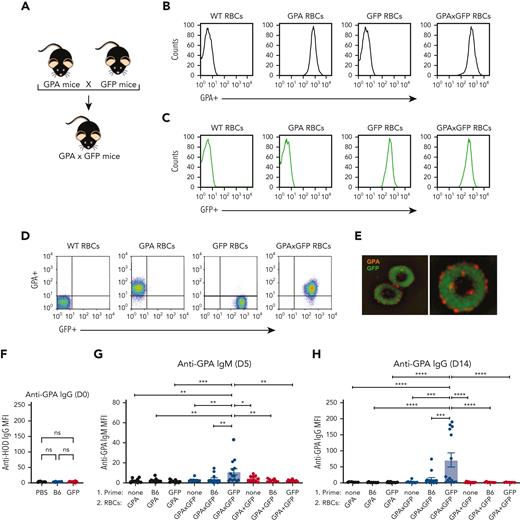
In contrast to HOD RBCs, GPA RBCs failed to induce alloantibodies in the absence of inflammation.69 Given that prior exposure to an intracellular alloantigen enhanced HOD alloimmumnization, we sought to determine whether priming to an intracellular antigen could convert what would otherwise be a nonresponder into a responder toward the GPA antigen. To test this, we crossed GPA transgenic mice with GFP transgenic mice to produce GPA × GFP RBC donor mice (Figure 6A-E). Consistent with our previous results using the HOD model, GFP immunization alone failed to cause any detectable difference in antibody reactivity toward GPA in GFP immunized or nonimmunized recipients (Figure 6F). Although GFP exposed to GPA RBCs or GPA + GFP RBCs largely failed to induce a significant anti-GPA response (Figure 6F and G), many recipients previously primed to GFP did form primed recipients anti-GPA antibodies after exposure to GPA × GFP RBCs (Figure 6G and H). These results suggest that prior priming toward an intracellular antigen may also possess the capacity to render recipients who would otherwise be nonresponders to be responsive to RBC-induced alloimmunization.
Priming to intracellular GFP facilitates antibody formation against the surface GPA RBC antigen after GPA × GFP RBC exposure. (A) Schematic of breeding used to produce GPA × GFP mice. (B) Flow cytometric analysis displayed as histograms of B6, GPA, GFP, and GPA × GFP RBCs after staining with anti-GPA antibody. (C) Detection of endogenous GFP expression via flow cytometric analysis of B6, GPA, GFP, and GPA × GFP RBCs. (D) Flow cytometric analysis, displayed as dot plots, of B6, GPA, GFP, and GPA × GFP RBCs stained with anti-GPA antibody. (E) Representative images of GPA × GFP RBCs (magnification ×100; right image shows single cell). (F) Anti-GPA antibody formation examined in PBS-treated, GFP-primed or B6-exposed recipients. (G-H) Anti-GPA IgM (day 5 after transfusion) (G) or IgG (day 14 after transfusion) (H), after GPA, GPA × GFP, or GPA + GFP RBC exposure. Error bars represent mean ± SD. All panels show combined data from experiments reproduced 3 times. ∗ P ≤ .05, ∗∗ P ≤ .01, ∗∗∗ P ≤ .001, ∗∗∗∗ P ≤ .0001. ns, no significance.
Priming to intracellular GFP facilitates antibody formation against the surface GPA RBC antigen after GPA × GFP RBC exposure. (A) Schematic of breeding used to produce GPA × GFP mice. (B) Flow cytometric analysis displayed as histograms of B6, GPA, GFP, and GPA × GFP RBCs after staining with anti-GPA antibody. (C) Detection of endogenous GFP expression via flow cytometric analysis of B6, GPA, GFP, and GPA × GFP RBCs. (D) Flow cytometric analysis, displayed as dot plots, of B6, GPA, GFP, and GPA × GFP RBCs stained with anti-GPA antibody. (E) Representative images of GPA × GFP RBCs (magnification ×100; right image shows single cell). (F) Anti-GPA antibody formation examined in PBS-treated, GFP-primed or B6-exposed recipients. (G-H) Anti-GPA IgM (day 5 after transfusion) (G) or IgG (day 14 after transfusion) (H), after GPA, GPA × GFP, or GPA + GFP RBC exposure. Error bars represent mean ± SD. All panels show combined data from experiments reproduced 3 times. ∗ P ≤ .05, ∗∗ P ≤ .01, ∗∗∗ P ≤ .001, ∗∗∗∗ P ≤ .0001. ns, no significance.
Discussion
These findings highlight a previously unappreciated mechanism of B-cell help after RBC transfusion, in which priming to an intracellular antigen could enhance antibody formation against a surface antigen. These results add to the growing evidence that nontarget antigen CD4 T–cell help may drive, or at least contribute to, RBC alloantibody formation.35 Prior studies, both among patients and animal models, have examined CD4 T cells directed specifically toward the same target antigen engaged by B cells.24,58-64,70 This approach has defined key T-cell epitopes of the CD4 T–cell response to RBC alloantigens24,58-61,63 while also serving as an important substrate to begin elucidating innate immune pathways and overall signaling events that may govern CD4 T–cell responses to allogeneic RBC transfusion.24,62,63,70 In addition, priming to an intracellular antigen also converted what is normally an immunologically nonresponsive outcome after GPA RBC transfusion into a GPA RBC–induced alloimmunization.69 The present data do not rule out an important contribution of general differences in baseline immune function that may influence CD4 T cells, B cells, and other immune constituents between responders and nonresponders.22,25,71-73 However, these findings do suggest that specific priming directed against intracellular antigens may also influence RBC alloimmunization risk.
Classically, enhancement of humoral responses through CD4 T–cell help occurs via the direct recognition of a major histocompatibility–peptide complex, presenting an epitope of a target protein that is also bound by a corresponding BCR.29,30 Various studies have also demonstrated a process termed as linked recognition, through which CD4 T cells may recognize a major histocompatibility–peptide complex containing a peptide epitope within an immunogenic protein that is directly linked to a nonrelated B-cell antigen.31-34 In contrast, the present results suggest that B-cell uptake as well as cytoplasmic content processing and presentation may similarly influence the types of peptides processed and, thereby, the CD4 T–cell help that can enhance alloimmunization. In addition to intracellular contents, similar uptake of surface antigens, such as alloantigen targets of prior alloimmunization events or even possible plasma-associated alloantigens adsorbed onto the RBC surface, may serve as important substrates for CD4 T–cell responses that enhance alloimmunization. Although alloantibodies may evanesce with persistent CD4 T–cell help in patients with historical alloantibodies,74 transfusion of RBCs expressing a target antigen of previously or currently detected alloantibodies may be avoided. As a result, intracellular alloantigens or alloantigens adsorbed onto the RBC surface that are not detected in routine clinical assays may play a fundamental role in the ability of prior priming events to increase RBC alloimmunization. These results, therefore, provide important insight into the possible impact of a distinct preexisting adaptive immune response that would not be detected clinically but could influence alloimmunization toward multiple RBC alloantigens.
The ability of GFP priming to enhance alloimmunization after exposure to HOD × GFP or GPA × GFP RBCs appears to be distinct from antigen or epitope spreading in the setting of autoimmunity and tumor immunotherapy.75,76 During antigen or epitope spread, the initial targeting of immunodominant epitopes can result in damage to the target antigen cells via antibody-mediated and/or cell-mediated mechanisms. This immune-mediated damage can result in the release of additional antigens, which can be phagocytosed by antigen presenting cells.75,76 Antigen uptake can facilitate antigen presenting cell activation, which in addition to damage-induced inflammation can enhance antigen presentation to and activation of CD4 T cells. The same antigen can also engage antigen-specific B cells, which with CD4 T–cell help, can differentiate into antibody secreting cells that produce corresponding antibodies. As this process unfolds, additional antigens and epitopes are exposed to additional B- and T-cell clones, which also work together to generate additional immune responses.75,76 This process has been described in patients with warm autoantibodies and may also reflect an additional mechanism of sensitization during alloimmunization.18,77,78 As this process continues, the breadth of antigens targeted over a period of time increases.75,76 In contrast, the present results suggest that preexisting priming events to an intracellular antigen not physically associated with the B-cell target antigen can still enhance a naïve B-cell response to a completely unrelated target antigen. This appears to occur in the setting of no preexisting ability of the primed response to directly engage the target cell. These findings not only have implications in RBC alloimmunization but may also be operative in autoimmunity as a complementary mechanism to the traditional views of antigen spread. Such a mechanism may be particularly important when considering that recent data suggesting that T-cell tolerance is a critical barrier to inadvertent B-cell responses against self, especially in the context of autoimmune hemolytic anemia.33,34 Although deleterious in the setting of autoimmunity, leveraging this mechanism may improve the expansion of desirable immune responses after vaccination or during cancer immunotherapy.
The mechanism by which B cells, which only possess a scant cytoplasmic rim, are able to acquire intracytoplasmic contents after target antigen engagement likely reflects trogocytosis. Prior studies demonstrated that BCR or T-cell receptor engagement can facilitate membrane incorporation of an engaged cell, offering a possible mechanism of acquiring cell surface molecules from neighboring cells that may regulate B-cell, T-cell, or other cellular responses.37,38 Because most studies have examined this phenomenon in the context of interactions between immune cells,37,38 the possible impact intracellular antigen acquisition has on subsequent immunity remained incompletely defined. Understanding this process is important because re-exposure to surface RBC antigens after an immune response toward those antigens has occurred can be largely avoided to reduce the likelihood of severe or delayed hemolytic transfusion reactions in this scenario.36 Thus, although prior immunity toward a surface antigen may modulate subsequent RBC alloimmunization, direct drivers of alloimmunization are more likely to reflect responses to antigens not detected clinically. Furthermore, because surface antigens often exist in large macromolecular complexes,79,80 enhancement resulting from prior immunization to one alloantigen may actually reflect B-cell engulfment of another surface antigen that is directly tethered to the initial alloantigen, thus reflecting the existing concept of linked recognition, as opposed to priming via a completely unrelated antigen. Thus, whether an unrelated antigen, especially a soluble intracellular protein, could enhance alloimmunization remained unknown. Indeed, nearly all previous studies have understandably exclusively examined CD4 T–cell responses to the same target antigen of the B cells.24,58-64,70
The ability of B cells to remove a target antigen mirrors the findings of recent reports, in which antibodies alone have been shown to likewise remove RBC alloantigens,54,67,81-87 suggesting that even the BCR itself may possess a similar activity. However, the ability of GFP priming to enhance alloimmunization against HOD × GFP RBCs does not appear to reflect an anti-GFP antibody–mediated process; these results do not rule out the possibility that antibodies directed against other alloantigens, including alloantigens adsorbed on the RBC surface, could contribute to RBC alloimmunization.68,88 Such a finding would be equally compelling as antibody responses directed against intracellular contents and/or adsorbed alloantigens are not routinely evaluated for clinically. Similarly, although anti-GFP antibodies did not appear to directly contribute to the boosted HOD response, detection of antibodies directed against intracellular antigens could also serve as a surrogate for CD4 T cells primed toward the same antigen. Such an evaluation could prove useful when seeking to develop a screening measure for patients most at risk for RBC alloimmunization. In summary, these results suggest that immune priming toward an intracellular alloantigen could contribute to RBC alloimmunization. Because this is a model system within inherent limitations, whether these results are reflective of the processes that occur clinically remains unknown. However, these findings suggest some underlying principles by which increased rates of alloimmunization may occur.
Acknowledgments
This work was partially supported by the Burroughs Wellcome Trust Career Award for Medical Scientists, the National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) R01 HL165975 and HL135575 (S.R.S.), NHLBI R01 HL154034 (C.M.A.), and NHLBI R01 HL132951 (J.E.H.), and NHLBI Institutional Training Grant 5K12HL141953-05 (R.J.). This research project was partially supported by the Emory University Integrated Cellular Imaging Core.
Authorship
Contribution: R.J., S.R.P., S.R.S., and C.M.A. designed the research study and conducted experiments; R.J., S.R.P., S.-C.W., K.P., M.C., M.V.-Z., D.A., A.B., and C.M.A. carried out and analyzed experiments; C.J.L., K.E.H., J.E.H., S.C.E., C.D.J., and P.E.Z. critically evaluated data, provided important insight into experimental design, and aided in data interpretation; R.J., S.R.P., S.R.S., and C.M.A. wrote the manuscript, which was additionally edited and commented on by the others.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sean R. Stowell, Joint Program in Transfusion Medicine, Brigham and Women’s Hospital, National Center for Functional Glycomics, Harvard Medical School, 630E New Research Building, 77 Avenue Louis Pasteur, Boston, MA 02115; e-mail: srstowell@bwh.harvard.edu; and Connie M. Arthur, Joint Program in Transfusion Medicine, Brigham and Women’s Hospital, National Center for Functional Glycomics, Harvard Medical School, 630D New Research Building, 77 Avenue Louis Pasteur, Boston, MA 02115; e-mail: cmarthur@bwh.harvard.edu.
References
Author notes
∗R.J. and S.R.P. contributed equally to this study.
Original data are available on request from the corresponding authors, Sean R. Stowell (srstowell@bwh.harvard.edu) or Connie M. Arthur (cmarthur@bwh.harvard.edu).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.