In this issue of Blood, Marín-Quílez et al identify novel functions of UDP-galactose-4-epimerase (GALE) that play a critical role in the glycosylation of maturated megakaryocytes and platelets.1
Growing experimental evidence suggests that genetic mutations affecting glycosylation and/or sialylation may contribute to abnormal platelet production and function. There are 2 types of glycosylation found in mammals: N-linked glycosylation transfers a mannose oligosaccharide to the asparagine residue within the sequon, whereas O-linked glycosylation occurs between N-acetylgalactosamine and serine or threonine residues during posttranslational modification. Hypoglycosylation results in congenital disorders of glycosylation (CDG) in humans. CDG patients frequently have altered hemostasis, such as thrombosis or bleeding complications, but the role of platelet glycosylation has not been rigorously explored.2 The biosynthesis of glycans (sugar trees) is usually terminated by the addition of sialic acid “cap” to prevent further chain elongation. Sialic acids are exposed on the platelet surface and regulate protein interactions, platelet aggregation, and adhesion. Aging platelets lose sialic acids from glycoproteins located on the membrane surface, forming desialylated platelets.3 The hepatic endocytic complex regulated by the Ashwell-Morell receptor removes these desialylated platelets from circulation.3 The abolished function of the sialyltransferase gene (St3gal4−/−) in mice results in thrombocytopenia, due to decreased sialylation and the exposure of terminal galactose residues on the platelet glycan surface.4 The GNE gene encodes a bifunctional enzyme, which controls sialic acid biosynthesis, and mutation of GNE causes thrombocytopenia. The β4GalT1 gene encodes a key enzyme involved in the addition of galactose moieties to glycoproteins. Mice transplanted with fetal liver-derived β4GalT1 knockout hematopoietic cells have profound thrombocytopenia, and in vitro cultivated mutant megakaryocytes are unable to produce proplatelets.5 Abolished Slc35a1 gene function also reduces sialylation in megakaryocytes and platelets, thereby inducing thrombocytopenia.6 Altogether, these results suggest that altered glycosylation, reduced sialic acid biosynthesis, or transfer to the sugar trees increases the number of desialylated platelets in the circulation, which is likely to be the major cause of reduced platelet life span and consequent thrombocytopenia in many CDG patients.
GALE encodes the UDP-galactose-4-epimerase enzyme, which regulates the final step of the Leloir galactose metabolic pathway, converting UDP-galactose to UDP-glucose. Abolished or dysregulated GALE functions result in an autosomal recessive form of type III galactosemia, leading to the accumulation of galactose and galactose-1-phosphate in the cell. CRISP-Cas9–mediated GALE deficiency in human cells triggers imbalanced nucleotide sugar content, reducing basal levels of UDP-N-acetylgalactosamine, thereby dramatically changing the process of glycoprotein and glycolipid biosynthesis. GALE deficiency also results in Fas hypoglycosylation and hypersensitivity to Fas ligand–induced cell death.7
Prior studies had already described rare GALE mutations with resultant hematologic defects in patients, including thrombocytopenia with dysplastic megakaryocytes.8,9 Reduced GALE expression with anti-GALE short hairpin RNA in CD34+-derived megakaryocyte precursors results in impaired megakaryocyte differentiation in vitro, suggesting that GALE plays a regulatory role in megakaryopoiesis and platelet production. Although GALE deficiency has been described in human patients, the exact in vivo pathologic mechanisms remain unclear, owing to the limited number of cases and the lack of humanized mouse model.
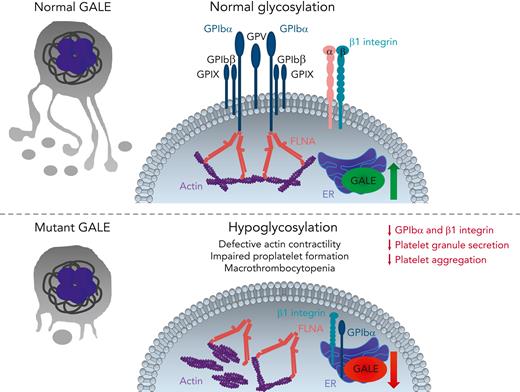
Marín-Quílez et al show that GALE is localized in the endoplasmic reticulum (ER) and that its expression is increased in mature megakaryocytes but reduced in platelet-like particles, indicating a restricted, time-dependent function of GALE during late steps of megakaryopoiesis (see figure). They identify 3 new GALE mutations in human patients with macrothrombocytopenia, associated with moderate to severe bleeding tendency. GALE-mutant patients had giant or gray platelets with aggregation and degranulation defects, which account for their bleeding diathesis. The enzymatic activity of UDP-galactose-4-epimerase and N-acetyl-lactosamine levels were strongly reduced, indicating abnormal glycosylation in megakaryocytes and platelets. GPIbα and β1 integrin were hypoglycosylated in GALE-mutant megakaryocytes, both proteins were mainly accumulated in the ER and cytoplasm, and externalization to the plasma membrane was strongly impaired. Defective glycosylation of GPIbα has been described in patients with Bernard-Soulier syndrome with consequent accumulation of GPIbα in the ER,2 and similar to GALE mutant platelets, only residual GPIbα protein was externalized. FLNA and GPIbα interaction regulates posttranslational assembly and trafficking of the GPIb-IX-V complex in megakaryocytes.10 In GALE-mutant megakaryocytes, the subcellular distribution of FLNA and actin was abnormal, supporting the idea that FLNA regulates the intracellular trafficking or GPIb-IX-V complex assembly and connects filamentous actins to GPIbα. Furthermore, GALE mutant megakaryocytes have reduced adhesion to von Willebrand factor, collagen, laminin, and fibronectin. These pathologic alterations translated to in vitro proplatelet formation defects in GALE-mutant megakaryocytes, suggesting that hypoglycosylation of adhesion receptors, impaired FLNA-mediated actin cytoskeleton, and GPIbα complex assembly may contribute to the reduced platelet production. Interestingly, GALE-mutant megakaryocytes were not apoptotic and maturation and ploidy were normal, but GALE-mutant platelets have increased levels of cleaved caspase 8, indicating that hypoglycosylated platelets are more prone to apoptosis.
GALE-mediated glycosylation in megakaryocytes and platelets. During late stages of megakaryopoiesis, GALE induces GPIbα and β1 integrin glycosylation in the ER, thereby leading to the trafficking of GPIb-IX-V complex and β1 integrin onto the megakaryocyte surface and subsequently onto newborn platelets. Normal glycosylation supports the interactions between FLNA and GPIbα in the plasma membrane and connects the actin cytoskeleton to the GPIbα complex. In contrast, decreased protein levels and/or reduced enzymatic activity of GALE leads to GPIbα and β1 integrin hypoglycosylation, retaining them in the ER and reducing their externalization on the cell surface. Impaired GALE function also leads to the disorganized and nonuniform actin cytoskeleton and delocalization of FLNA and actin from the plasma membrane to the cytoplasm, forming actin patches, thereby reducing actin contractility. These pathologic processes strongly impair platelet production, size, and function. Therefore, GALE mutant-patients develop macrothrombocytopenia, characterized by giant/gray and apoptotic platelets with strongly reduced membrane localization of GPIb-IX-V complex and β1 integrin, defective granule secretion, and platelet aggregation. GP, glycoprotein; FLNA, filamin A.
GALE-mediated glycosylation in megakaryocytes and platelets. During late stages of megakaryopoiesis, GALE induces GPIbα and β1 integrin glycosylation in the ER, thereby leading to the trafficking of GPIb-IX-V complex and β1 integrin onto the megakaryocyte surface and subsequently onto newborn platelets. Normal glycosylation supports the interactions between FLNA and GPIbα in the plasma membrane and connects the actin cytoskeleton to the GPIbα complex. In contrast, decreased protein levels and/or reduced enzymatic activity of GALE leads to GPIbα and β1 integrin hypoglycosylation, retaining them in the ER and reducing their externalization on the cell surface. Impaired GALE function also leads to the disorganized and nonuniform actin cytoskeleton and delocalization of FLNA and actin from the plasma membrane to the cytoplasm, forming actin patches, thereby reducing actin contractility. These pathologic processes strongly impair platelet production, size, and function. Therefore, GALE mutant-patients develop macrothrombocytopenia, characterized by giant/gray and apoptotic platelets with strongly reduced membrane localization of GPIb-IX-V complex and β1 integrin, defective granule secretion, and platelet aggregation. GP, glycoprotein; FLNA, filamin A.
The study by Marín-Quílez et al provides new insights into the GALE-mediated glycosylation mechanisms in megakaryocytes and platelets. GALE mutation not only dysregulates platelet structure and functions but also reduces platelet production in these patients. Future studies are required to further investigate the role of defective glycosylation and altered FLNA function in GALE-mutant megakaryocytes and platelets. It is important to analyze GALE-dependent glycosylation patterns on platelet surface proteins, which are involved in platelet aggregation and thrombus formation, in the assembly of procoagulant complexes, and in the process of blood clotting. Abnormal glycosylation of coagulation factors may also dysregulate hemostasis, thereby inducing bleeding complications in GALE-mutant patients. Finally, the generation of humanized mouse models mimicking GALE mutations would help to identify novel GALE-mediated mechanisms in megakaryocytes and platelets and develop new therapeutic approaches.
Conflict-of-interest disclosure: The authors declare no competing financial interests.