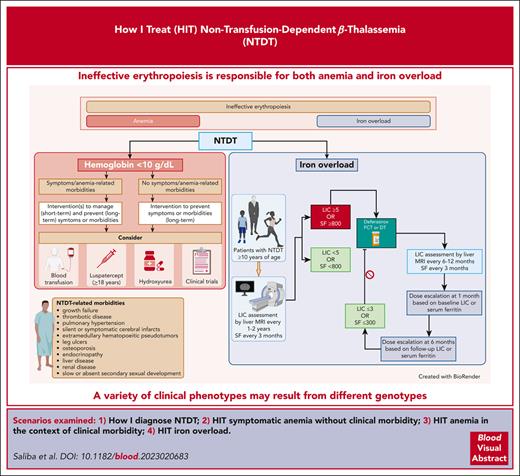
Abstract
The intricate interplay of anemia and iron overload under the pathophysiological umbrella of ineffective erythropoiesis in non-transfusion-dependent β-thalassemia (NTDT) results in a complex variety of clinical phenotypes that are challenging to diagnose and manage. In this article, we use a clinical framework rooted in pathophysiology to present 4 common scenarios of patients with NTDT. Starting from practical considerations in the diagnosis of NTDT, we delineate our strategy for the longitudinal care of patients who exhibit different constellations of symptoms and complications. We highlight the use of transfusion therapy and novel agents, such as luspatercept, in the patient with anemia-related complications. We also describe our approach to chelation therapy in the patient with iron overload. Although tackling every specific complication of NTDT is beyond the scope of this article, we touch on the management of the various morbidities and multisystem manifestations of the disease.
Introduction
Since the previous How I Treat articles focusing on the thalassemias were published in Blood in 2011 and 2018, our armamentarium for the management of these disorders has expanded.1,2 In this article, we present 4 cases as examples of the most contemporary clinical approaches to the diagnosis and management of non-transfusion-dependent β-thalassemia (NTDT), specifically β-thalassemia intermedia. Although some aspects of care continue to be extrapolated from transfusion-dependent β-thalassemia (TDT), the approach for patients with NTDT should be individualized while considering opportunities for interventions with the potential of improving short-term or long-term outcomes, such as blood transfusion, iron chelation therapy (ICT), hydroxyurea, and luspatercept. The distinction between TDT and NTDT may vary with time because a patient with NTDT may increasingly depend on regular transfusion support or a patient with TDT may require a less intensive transfusion program with certain interventions.3
Patient 1: diagnosing non-transfusion-dependent β-thalassemia, the intricate interplay of genotype and phenotype
A 25-year-old woman was referred to our clinic for moderate chronic microcytic anemia. The hemoglobin (Hb) concentration was 9.5 g/dL, and the mean corpuscular volume was 69 fL. The red-cell distribution width was normal, and the mean corpuscular Hb concentration was low. Before she was referred to us, she was initially treated empirically for iron deficiency anemia with oral ferrous sulfate without improvement. Lactate dehydrogenase level, reticulocyte count, and indirect bilirubin level were mildly elevated; haptoglobin was suppressed. Hb electrophoresis showed an elevated HbA2 fraction at 4.5% and a normal HbF fraction at 0.7%. She was initially thought to have a severe manifestation of heterozygous β-thalassemia. Her ferritin level was elevated at 780 ng/mL. α-Globin gene analysis demonstrated the presence of an additional α-globin gene in a DNA insert of 3.7 kb. This patient had β-thalassemia intermedia, resulting from increased α/β-globin chain imbalance in the setting of 1 defective β-globin gene (IVSI-110 G>A) and a triplicated α-globin gene locus.
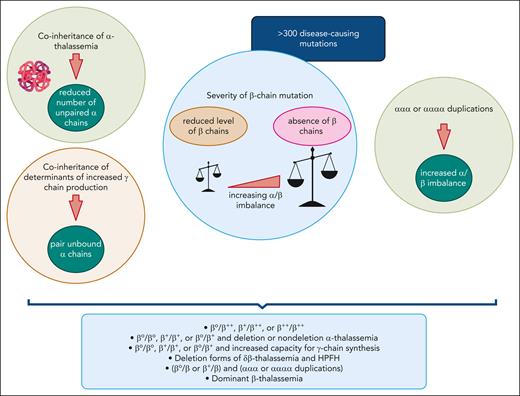
In β-thalassemia intermedia, as the name implies, the disease is characterized by a clinical severity that is intermediate when compared with β-thalassemia major and the asymptomatic carrier (trait/minor) state. Practically, this usually manifests as delayed age at presentation (commonly >2 years of age) with mild-to-moderate anemia (commonly 7-10 g/dL).4-7 The β-thalassemia intermedia phenotype can result from a variety of genetic alterations that lead to an α/β-globin chain imbalance of sufficient magnitude to promote ineffective erythropoiesis and subsequent anemia of such intermediate severity. This includes various homozygous or compound heterozygous states for β-thalassemia mutations or in some instances, such as the patient described in this study, heterozygous β-thalassemia mutations combined with α-globin gene duplications. The variety of mutations that may affect the β-globin gene in the homozygous or compound heterozygous state is the primary modifier of phenotype. These β-globin gene mutations include mild promoter mutations that cause a mild reduction in β-globin chain synthesis (β++), β0 states in which there is complete absence of β-globin chain production, and β+ mutations of intermediate severity.6,8,9 Even patients with homozygous β0 mutations can present with a β-thalassemia intermedia phenotype due to secondary genetic modifiers with attenuating effects on the α/β-globin chain imbalance, such as coinheritance of α-thalassemia or hereditary persistence of fetal hemoglobin. The structural hemoglobin variant hemoglobin E also behaves similar to a mild β-globin gene mutation, and patients with hemoglobin E/β-thalassemia can have a similar phenotype as that of β-thalassemia intermedia (often considered as a genetic subtype), with similar genetic modifiers.5,6,10Figure 1 depicts the interplay among primary, secondary, and tertiary genetic modifiers of phenotype in β-thalassemia intermedia.6,7,11-13 Supplemental Figure 1 (available on the Blood website) shows the diagnostic approach to thalassemia. Variants in NTDT include β-thalassemia intermedia and mild-to-moderate hemoglobin E/β-thalassemia. Although patients with hemoglobin S/β-thalassemia and hemoglobin C/β-thalassemia may have similar clinical needs to those of patients with NTDT, these diseases have unique characteristics and will not be covered in this article. Most of the evidence upon which we based our approach to NTDT focuses on β-thalassemia intermedia.
Modifiers of the β-thalassemia phenotype leading to β-thalassemia intermedia. The primary modifier of phenotype is the severity of the β-globin chain mutation (β++, β+, or β0), with varying α/β-globin chain imbalance. Coinheritance of α-thalassemia and increased expression of α-globin stabilizing protein act as secondary modifiers that mitigate the α/β-globin chain imbalance. In contrast, inheritance of a triplicated or quadruplicated α-globin gene locus may amplify the severity of a heterozygous β-thalassemia state. In some instances, a single dominantly inherited β-globin locus mutation can be responsible for a β-thalassemia intermedia phenotype. HPFH, hereditary persistence of fetal hemoglobin. Figure created with BioRender.
Modifiers of the β-thalassemia phenotype leading to β-thalassemia intermedia. The primary modifier of phenotype is the severity of the β-globin chain mutation (β++, β+, or β0), with varying α/β-globin chain imbalance. Coinheritance of α-thalassemia and increased expression of α-globin stabilizing protein act as secondary modifiers that mitigate the α/β-globin chain imbalance. In contrast, inheritance of a triplicated or quadruplicated α-globin gene locus may amplify the severity of a heterozygous β-thalassemia state. In some instances, a single dominantly inherited β-globin locus mutation can be responsible for a β-thalassemia intermedia phenotype. HPFH, hereditary persistence of fetal hemoglobin. Figure created with BioRender.
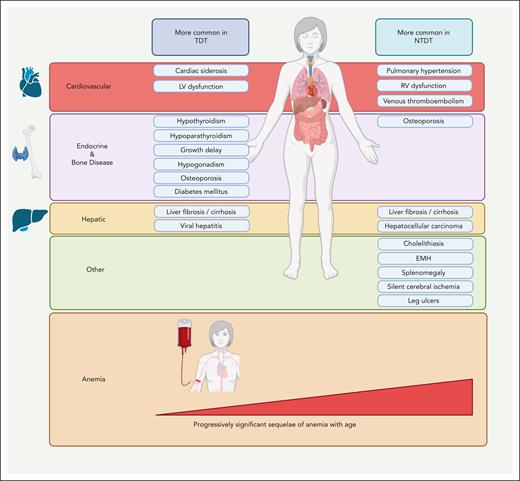
In the past decade, patients with β-thalassemia intermedia have been recognized as having NTDT to emphasize their independence from requiring lifelong transfusions for survival, compared with patients with β-thalassemia major.3,4,6 However, such transfusion independence did not come without its own side effects, and it has recently become apparent that untreated ineffective erythropoiesis can lead to various clinical morbidities attributed to chronic anemia and hemolysis, primary iron overload, and hypercoagulability.14 Altogether, this leads to a somewhat different morbidity profile than that seen in TDT (β-thalassemia major), as illustrated in Figure 2.5,6 As will be illustrated in the next cases, intermittent or regular transfusions may still be used for patients with NTDT to prevent or manage clinical sequelae at certain points in time. When management decisions and assessment for eligibility for clinical trials rely on the “real-time” status, we usually consider patients as having NTDT if they had received <6 red blood cell units in the past 6 months and zero transfusions in the preceding 2 months.
Complications of NTDT compared with those of TDT. The prevalence of clinical morbidities in TDT and NTDT is based on observational studies. The figure illustrates those complications that are observed at a higher prevalence in NTDT or TDT, although all mentioned complications can manifest in both entities. The prominence of uncontrolled ineffective erythropoiesis in the absence of transfusion therapy mediates a distinctive constellation of complications related to chronic anemia, hypercoagulability, and iron overload in NTDT. EMH, extramedullary hematopoiesis; LV, left ventricular; RV, right ventricular. Figure created with BioRender.
Complications of NTDT compared with those of TDT. The prevalence of clinical morbidities in TDT and NTDT is based on observational studies. The figure illustrates those complications that are observed at a higher prevalence in NTDT or TDT, although all mentioned complications can manifest in both entities. The prominence of uncontrolled ineffective erythropoiesis in the absence of transfusion therapy mediates a distinctive constellation of complications related to chronic anemia, hypercoagulability, and iron overload in NTDT. EMH, extramedullary hematopoiesis; LV, left ventricular; RV, right ventricular. Figure created with BioRender.
The clinical presentation of this patient emphasizes the importance of full genotyping in patients with presentations consistent with β-thalassemia intermedia phenotypes. Clinical clues include microcytic anemia that is out of proportion to the extent of abnormalities in the β-globin chains or an elevated ferritin in the absence of an alternative explanation for anemia, such as inflammation or chronic disease.15,16 Hb electrophoresis or high-performance liquid chromatography can confirm a clinical suspicion of NTDT. We subsequently ascertain the genotype via DNA analysis of both the α- and β-globin chains. An accurate diagnosis is important for appropriate follow-up, counseling, and management, as we highlight in the subsequent cases.
When NTDT is diagnosed in children, careful assessment of management needs is essential, especially to avoid unnecessary long-term transfusion therapy. Although children can present with severe anemia during periods of infection for example, transfusion needs may significantly vary between such periods of stress and steady state.17,18
Patient 2: symptomatic anemia without clinical morbidity
Patient 2 is a 40-year-old man who was diagnosed with β-thalassemia intermedia (heterozygous IVSI-6 T>C/β codon 44 [−C]) when he was 7 years old. The clinical phenotype had been most consistent with NTDT. His Hb concentration ranged between 9.5 and 10.5 g/dL throughout the first 3 decades of his life. Over the past 3 years, his anemia had worsened, with a Hb concentration ranging between 9.0 and 9.5 g/dL. His fatigue was affecting his job as a construction worker. He subsequently developed dyspnea on exertion. His echocardiogram showed a hyperdynamic left ventricle and a normal right ventricular systolic pressure. His electrocardiogram and thyroid function testing showed normal results. Dual-energy radiograph absorptiometry showed osteopenia. Liver enzyme testing showed mildly elevated alanine aminotransferase and indirect bilirubin levels. Liver elastography showed no fibrosis. Serum ferritin was 520 ng/mL. Liver iron concentration (LIC) via R2 magnetic resonance imaging (MRI) was estimated at 4.8 mg Fe per gram dry weight (dw). To manage his mild yet symptomatic anemia, we were hesitant to start the patient on regular transfusion therapy, considering the risks of secondary iron overload and complications. He also declined hydroxyurea therapy because of concerns about fertility. We still needed to initiate an intervention considering his symptoms and future risk of morbidity associated with an Hb level <10 g/dL. We eventually offered him to consider an active clinical trial evaluating luspatercept for improvement of Hb concentration in adult patients with NTDT.
Chronic anemia resulting from ineffective erythropoiesis is associated with end-organ damage in children and adults with NTDT.19-21 An Hb level of ≥10 g/dL is associated with a longer overall survival and is predictive of the absence of NTDT-specific complications, including liver disease, endocrinopathies, bone disease, leg ulcers, extramedullary hematopoiesis, thromboembolism, and pulmonary hypertension.22-24 An improvement in Hb level by 1 g/dL has also been linked to lower rates of NTDT-specific morbidity.25 Patients with an Hb level <10 g/dL are likely to benefit from therapy and, when possible, should be considered for an intervention or clinical trial to prevent future risk of serious morbidity development.5,14,22,25,26 Splenectomy has been historically used to increase the Hb level by 1 or 2 g/dL, but the procedure is now less commonly used, owing to the risks of infection and thrombosis.20,27-30 Splenectomy is mainly reserved for cases of splenomegaly or hypersplenism.4 Hydroxyurea has also shown some benefit in small studies of patients with NTDT. However, data were not as encouraging as in patients with sickle-cell disease, and long-term safety and durability of response remain a concern.31,32 The benefit of hydroxyurea is often most pronounced in patients with the XmnI polymorphism, rs766432 polymorphism at intron 2 of the BCL11A gene, Hb Lepore, δβ-thalassemia, or HbE β-thalassemia.31,33-35 When used, we start hydroxyurea at 10 mg/kg per day and escalate it by 3 or 5 mg/kg per day every 8 weeks to a maximum of 20 mg/kg per day. Transfusions may theoretically be used, but the risk of secondary iron overload and need for ICT for long-term treatment cannot be ignored.20 Although avoiding transfusional iron loading is important, it is equally important to recognize the accelerated primary iron overload, increased hypercoagulability, and accentuated anemia-related hypoxia resulting from ineffective erythropoiesis.36-39 Beyond the effects of hypoxia on organs, chronic anemia has been linked to compromised quality of life, mental health, and overall well-being.14,40-43 Because our clinical toolbox is further enriched with novel therapies, we have attempted to look beyond the immediate effects of chronic anemia to its implications on long-term morbidity, mortality, and quality of life.
Luspatercept is a recombinant protein, with a modified extracellular domain of the human activin receptor type IIB fused to the Fc domain of human immunoglobulin G1.44,45 Luspatercept binds to transforming growth factor β superfamily ligands and stimulates erythroid maturation by blocking SMAD2/3 signaling.44,45 Luspatercept is approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of TDT.46 Luspatercept is not currently approved by the FDA for the treatment of NTDT but received approval from the EMA for adults with NTDT in March 2023.47 To date, the strongest evidence supporting the use of luspatercept in NTDT comes from the phase 2, double-blind, randomized, placebo controlled BEYOND study (NCT03342404), evaluating the efficacy and safety of luspatercept in patients with NTDT and Hb concentration of ≤10 g/dL.48 Luspatercept was associated with a significant improvement in Hb concentration by ≥1 g/dL over a continuous 12-week period from weeks 13 to 24 of therapy without transfusion support.48 Luspatercept therapy also resulted in the improvement of weakness and tiredness, commensurate with improvements in Hb levels.48 In countries where luspatercept is approved for this indication, we support its use in patients with NTDT with Hb <10 g/dL, especially those with anemia-related symptoms, such as weakness and fatigue. We simultaneously await the data reporting the long-term effects of luspatercept on NTDT-related morbidities. We fully acknowledge and recognize the anticipated financial challenges associated with drug pricing and affordability, particularly in low-to-middle–income countries. We understand the importance of addressing inequalities in access to crucial interventions and treatments for patients in these settings.
In addition to the erythroid maturation agent luspatercept, clinical studies are currently investigating other pharmacologic agents for their ability to improve anemia in NTDT, such as the pyruvate kinase activators mitapivat (NCT04770753 and NCT03692052) and etavopivat (NCT04987489).49 Because serum ferritin level was <800 ng/mL and LIC was <5 mg Fe per gram dw, our patient was not started on ICT.4,5
Regarding this patient’s osteopenia, despite the lack of mechanistic studies specifically in NTDT, bone disease in β-thalassemia is likely multifactorial, with ineffective erythropoiesis, iron overload, hypogonadism, hypoparathyroidism, and bone marrow expansion playing a role in the pathophysiology.50,51 Bone marrow expansion leads to medullary trabecular destruction and cortical thinning,52 and suppressed hepcidin favors bone resorption over formation.53,54 Because data regarding therapy from interventional trials are lacking in the context of NTDT, we recommend vitamin D supplementation in the absence of contraindications and offer bisphosphonate therapy for patients with fragility fractures, osteoporosis, or osteopenia with a high risk of fracture.55,56
Patient 3: anemia in the context of clinical morbidity
Our next patient is a 32-year-old woman with a diagnosis of β-thalassemia intermedia (β0/β+ and deletional α-thalassemia) since the age of 5 years, when she presented with microcytic anemia and an Hb concentration of 8.3 g/dL. She underwent splenectomy 2 years after her diagnosis and did not experience any periods of developmental or growth delays. She had received minimal transfusion, with a lifetime total of 4 units of packed red blood cells around the time of a laparoscopic appendectomy 5 years ago. She reported dyspnea while working as a nurse in a pediatric clinic. She had a 7 mm superficial ulcer over the right medial malleolus. Her Hb concentration was 7.8 g/dL, with mean corpuscular volume of 59 fL, and the platelet count was elevated at 920 × 109/L. Serum ferritin level was 450 ng/mL. LIC via R2 MRI was estimated at 7.2 mg Fe per gram dw. Transthoracic echocardiography showed a tricuspid regurgitation velocity of 3.4 m/s (normal, <2.5 m/s) and a left ventricular ejection fraction of 65% without any other abnormalities. Cardiac MRI with T2∗ sequence was normal (T2∗, >20 ms). After referral to the multidisciplinary pulmonary hypertension clinic, right cardiac catheterization confirmed the presence of pulmonary hypertension (PH), with an elevated mean pulmonary arterial pressure of 50 mmHg (normal, <20 mmHg). Radionuclide ventilation/perfusion scan revealed normal findings. She also reported persistent lower back pain radiating from her lower back down to her right thigh and knee.
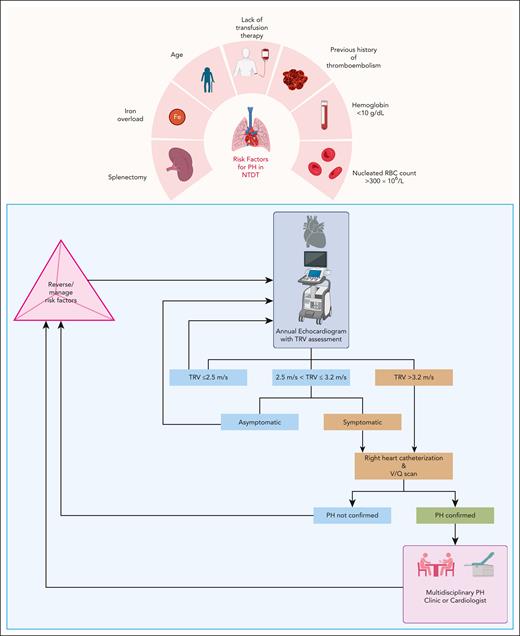
This patient’s journey with NTDT showcases the perils of chronic ineffective erythropoiesis and anemia. Starting with her most clinically significant comorbidity, her PH was addressed first. The updated clinical classification of PH by the World Health Organization shifted PH associated with β-thalassemia to group 5, a potpourri category of conditions associated with PH with unexplained/multifactorial mechanisms.57 Historically, PH associated with NTDT was considered as part of group 1 PH because it is frequently characterized by precapillary pulmonary arterial hypertension with the absence of left-sided heart disease, pulmonary disease, and chronic thromboembolism. However, given our limited understanding of the multiple factors underpinning PH in NTDT, its pathophysiology remains unclear and could include transfusion independence, hypercoagulability, and thromboembolism in the pulmonary vasculature, especially in adults who underwent splenectomy, chronic anemia, hypoxia with high cardiac output, left-heart disease, iron overload and endothelial damage, inactivation of nitric oxide by free plasma Hb, and hyperviscosity.22,28,58,59 PH is one of the leading causes of death in NTDT.60 In patients with PH confirmed via cardiac catheterization, the rate of mortality could reach up to 40%, with a median survival of 9 years.61
Our approach to screening for PH in NTDT and the subsequent management is summarized in Figure 3.2,4 We focus on a multidisciplinary approach, with the involvement of a specialized PH clinician or simultaneous involvement of a cardiologist, a pulmonologist, and a hematologist. PH symptoms can limit function and affect the quality of life in patients with NTDT.59 In the absence of evidence from randomized controlled trials for pharmacologic agents targeting PH in thalassemia, we focus our efforts on reversing and managing the risk factors that have been identified in observational studies.28,59 We typically start an individualized short-term regular transfusion therapy program in patients with PH and other morbidities, such as leg ulcers or extramedullary hematopoiesis in which there is evidence of benefit, albeit from observational studies.4,20,62 In fact, patients with NTDT who transitioned to receive regular transfusion programs for management of morbidities had longer cardiovascular disease–related survival than patients who did not.60 There is some evidence to the benefit for hydroxyurea therapy in this setting, particularly in the patient with thrombocytosis.31,63,64 Starting patients on antiplatelet therapy would also be advised.4 In patients with iron overload, we also start ICT in patients with LIC ≥5 mg Fe per gram dw or serum ferritin level ≥800 ng/mL, for which LIC measurement is not available.4,5 We discontinue ICT when LIC <3 mg Fe per gram dw or serum ferritin level <300 ng/mL.4,5
Risk factors for PH and management approach in patients with NTDT. Patients with NTDT should be screened annually with a transthoracic echocardiogram with assessment of the tricuspid regurgitant velocity (TRV). For patients who are symptomatic or have a TRV >3.2 m/s, right heart catheterization is appropriate to confirm the diagnosis of PH. Other secondary causes should be excluded. A ventilation/perfusion (V/Q) scan should be obtained to evaluate for chronic thromboembolic disease. If asymptomatic and 2.5 < TRV ≤ 3.2 m/s, close follow-up is warranted, and management of reversible risk factors should be considered. RBC, red blood cell. Figure created with BioRender.
Risk factors for PH and management approach in patients with NTDT. Patients with NTDT should be screened annually with a transthoracic echocardiogram with assessment of the tricuspid regurgitant velocity (TRV). For patients who are symptomatic or have a TRV >3.2 m/s, right heart catheterization is appropriate to confirm the diagnosis of PH. Other secondary causes should be excluded. A ventilation/perfusion (V/Q) scan should be obtained to evaluate for chronic thromboembolic disease. If asymptomatic and 2.5 < TRV ≤ 3.2 m/s, close follow-up is warranted, and management of reversible risk factors should be considered. RBC, red blood cell. Figure created with BioRender.
Our patient was treated with a regular transfusion regimen of 2 units of packed red blood cells every 4 weeks, to maintain (pretransfusion) Hb level ≥10 g/dL. We recommend using leukoreduced and Rh- (C, c, E, and e) and Kell-matched red blood cell products to mitigate the risk of alloimmunization in all patients with NTDT and especially in patients who were pregnant, underwent splenectomy, and received minimal transfusion.4
Our patient’s functional capacity improved. Her leg ulcer also gradually healed. She was concomitantly started on aspirin and ICT with deferasirox film–coated tablets. We use aspirin in patients with thrombocytosis (≥500 × 109/L) and history of splenectomy, which are 2 risk factors for thrombosis in NTDT. We base this clinical decision on retrospective data showing a lower risk of recurrence with aspirin therapy in patients with NTDT after the index thrombotic event.65,66 The absence of risk factors for provoked venous thromboembolism underlines the unique pathophysiology of hypercoagulability in NTDT, with increased endothelial and platelet activation and changes to the lipid membrane composition in abnormal red blood cells among other factors.67-69
When we attempted to decrease the frequency of transfusion therapy, her Hb concentration dropped to the range from 7 to 8 g/dL. Given that she had developed a complication related to her anemia, we maintained a regular transfusion regimen every 4 weeks. In this context and after 2 years of regular transfusion therapy, we started approaching her care as a patient with TDT. Luspatercept was eventually deployed and up-titrated to a dose of 1.25 mg/kg administered every 21 days. With luspatercept therapy, we were able to bring down her transfusion requirement to 2 units of packed red blood cells every 8 weeks.46 ICT was continuously adjusted per guidance in patients with TDT.
MRI of the lumbosacral spine showed paraspinal masses consistent with extramedullary hematopoiesis compressing the nerve roots of L4 and L5 on the right. Those masses decreased in size, and her pain improved with the blood transfusion regimen mentioned earlier.
Patient 4: iron overload
A 37-year-old man was diagnosed with β-thalassemia intermedia when he was 4 years old. He only required 1 transfusion of packed red blood cells at age 8 years. He presented at the clinic to establish care. His Hb concentration was 8.4 g/dL, and his serum ferritin level was 940 ng/mL. MRI of the liver with T2∗ sequences estimated the LIC at 8.2 mg Fe per gram dw. Cardiac MRI with T2∗ sequences yielded a normal T2∗ of 28 ms (normal, >20 ms). Three months before this visit, he was diagnosed by his primary care physician with hypothyroidism, and levothyroxine replacement was prescribed.
In NTDT, hypoxia and ineffective erythropoiesis lead to an inappropriate suppression of hepcidin, which stimulates intestinal iron absorption, promotes release of recycled iron from the reticuloendothelial system, and accelerates primary iron overload even in the absence of transfusions.36,71-73 Hypoxia also activates the EPOR/JAK2/STAT5 pathway and upregulates the secretion of erythroferrone by erythroblasts leading to the inhibition of hepcidin production in the liver.74,75 As mentioned earlier in this article, the severity of chronic anemia correlates with the extent of primary iron overload.38,39 In NTDT, iron deposition favors the liver over the myocardium, with an increased LIC accompanied by a relatively modest increase in serum ferritin level.76,77 Patients with TDT, in contrast, develop secondary iron overload as a result of regular transfusional iron loading.77,78 Iron accumulation in patients with NTDT is cumulative and is associated with a high risk of hepatic disease and cancer, thrombosis, PH, hypothyroidism, osteoporosis, and hypogonadism.78-82 Secondary siderosis can also contribute to iron overload in patients with NTDT receiving transfusion therapy.
Early detection of iron overload is important to facilitate early intervention, prevent further iron accumulation, and reverse previous iron deposition. The preferred noninvasive method for diagnosis and monitoring of iron overload in NTDT is using a liver MRI with R2 or T2∗ techniques.4 Because NTDT is prevalent in areas of the world where advanced MRI techniques may not be available, serum ferritin level is used as a marker of iron overload when LIC measurement is not possible. Spot serum ferritin measurement can underestimate the degree of iron overload in many patients with NTDT because the ratio of serum ferritin level to LIC is lower than that in TDT.83 However, the trends in serum ferritin levels over time remain informative and can be followed in the absence of access to a liver MRI. We typically start screening patients with NTDT for iron overload as of age 10 years.79 We perform a baseline MRI for LIC measurement and repeat it every 1 or 2 years in the absence of indications for ICT. For patients receiving ICT, we track the LIC via MRI every 6 or 12 months. In the context of NTDT, there is insufficient evidence to recommend routine screening with cardiac MRI T2∗.4,78
Deferasirox is the only iron chelator that is specifically approved by the FDA and EMA as a first-line iron chelator in patients with NTDT who are aged ≥10 years, based on data from the THALASSA trial.84,85 Experience with other iron chelators, such as deferoxamine and deferiprone, is limited.86 A novel and more highly bioavailable formulation of deferasirox in the form of a film-coated tablet is available and offers an alternative, often easier, and more palatable way to administer ICT without the need for preparation or mixing.87 Patients with a LIC ≥5 mg Fe per gram dw or serum ferritin level ≥800 ng/mL are at the highest risk for iron-related morbidity and mortality; therefore, we recommend starting ICT, typically with deferasirox, when these thresholds are met.23,88,89 Even in patients with a serum ferritin levels from 300 to 800 ng/mL (and no MRI assessment available), ICT may be considered because the probability of LIC being >5 mg Fe per gram dw is still high, especially in patients with other evidence of iron overload or related morbidity.4,90 Although maximum deferasirox doses of 20 mg/kg per day (dispersible tablet) were used in the THALASSA trial, higher doses up to 30 mg/kg per day may be needed to achieve target iron goals, as noted in the THETIS trial.91 ICT can be interrupted when LIC or serum ferritin level reach 3 mg Fe per gram dw or 300 ng/mL, respectively.4 Although overall survival was not assessed as an outcome in the landmark trials, the use of ICT was associated with a decreased risk of death from liver disease in a large cohort of patients with NTDT.60 When the use of deferasirox is contraindicated or unavailable, deferiprone or deferoxamine may be used based on data from an Italian study in β-thalassemia intermedia.86,92-94 Safety monitoring should follow local prescribing information.93
Returning to our patient, MRI assessment was consistent with hepatic iron overload but not myocardial iron overload. ICT with oral deferasirox was started; the film-coated tablet formulation was chosen for ease of administration at a dose of 14 mg/kg per day. At 6 months, LIC was 6.4 mg Fe per gram dw, and the serum ferritin level was 820 ng/mL, so we continued the same dose with steady improvement in LIC at 1-year follow-up. Hypothyroidism was likely an endocrine manifestation of iron overload in this case.
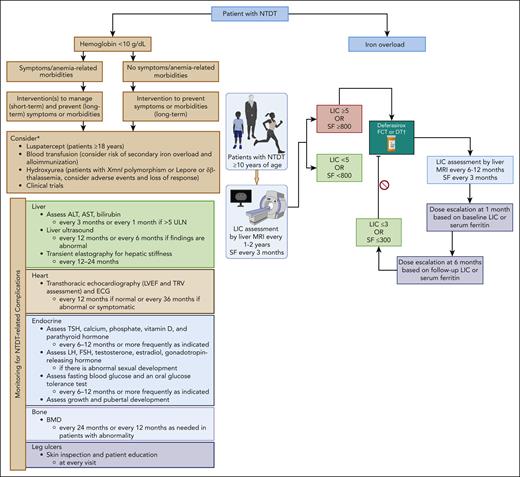
To summarize key insights from the last 4 cases, our approach to the assessment and management of anemia and iron overload as part of the overall management of NTDT are summarized in Figure 4.4
Approach to the assessment and management of anemia and iron overload in NTDT. The assessment and management of NTDT focuses on the manifestations and severity of anemia and iron overload. The general management of patients with NTDT is summarized. ∗Luspatercept is only approved for NTDT in the European Union. Dosing and monitoring of luspatercept and hydroxyurea should follow local prescribing information. The choice and duration of therapy (used for prevention or management of adverse outcomes) should rely on overall patient well-being, symptoms, comorbidities, access, and patient preference. †Deferasirox dosing and safety monitoring to follow local prescribing information. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMD, bone mass densitometry; DT, dispersible tablet; ECG, electrocardiogram; FCT, film-coated tablet; FSH, follicle stimulating hormone; LH, luteinizing hormone; LVEF, left ventricular ejection fraction; SF, serum ferritin in ng/mL; TSH, thyroid stimulating hormone; ULN, upper limit of normal. Figure created with BioRender.
Approach to the assessment and management of anemia and iron overload in NTDT. The assessment and management of NTDT focuses on the manifestations and severity of anemia and iron overload. The general management of patients with NTDT is summarized. ∗Luspatercept is only approved for NTDT in the European Union. Dosing and monitoring of luspatercept and hydroxyurea should follow local prescribing information. The choice and duration of therapy (used for prevention or management of adverse outcomes) should rely on overall patient well-being, symptoms, comorbidities, access, and patient preference. †Deferasirox dosing and safety monitoring to follow local prescribing information. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMD, bone mass densitometry; DT, dispersible tablet; ECG, electrocardiogram; FCT, film-coated tablet; FSH, follicle stimulating hormone; LH, luteinizing hormone; LVEF, left ventricular ejection fraction; SF, serum ferritin in ng/mL; TSH, thyroid stimulating hormone; ULN, upper limit of normal. Figure created with BioRender.
Practical challenges and final thoughts
As we now understand that untreated anemia carries detrimental long-term consequences in patients with NTDT, research and development are being geared toward finding the best approach to therapy. Although transfusions and agents such as hydroxyurea have been around and used in other hemoglobinopathies for decades, they may not necessarily specifically address the unmet need in this patient population, which not only requires anemia correction but also demands a treatment option that is safe and durable for such a chronic disease. Amidst the excitement about upcoming novel therapies, we are cognizant of the limited affordability and access to novel therapies in many regions of the globe where the disease is most prevalent.
Because patients with NTDT are living longer and experiencing longer-term morbidities than previously, it is imperative to establish a timely and appropriate diagnosis to allow preventive care and appropriate risk mitigation. Unlike patients with TDT who receive frequent follow-up as a byproduct of the need for frequent and regular transfusion therapy, patients with NTDT may not be easily identified or appropriately monitored. Engaging primary care providers in areas of higher prevalence may help early identification of patients, before the onset of morbidities. In parallel, developing the local expertise and coalescing efforts toward the development of national and regional centers of excellence can foster the advancement of clinical trials and guide local communities and clinics in the care of patients with NTDT.
With NTDT being a disease mostly diagnosed in childhood, the transition from pediatric to adult clinics is fraught with its own hurdles. Therefore, we recommend starting the process early, involving both the parent and the patient in the conversation, empowering the child or adolescent to assume progressive responsibility, and harnessing the expertise of the multidisciplinary team, including the nurse, the social worker, the pediatric hematologist, the adult hematologist, the psychologist when needed, and the financial counselor when applicable.
Authorship
Contribution: A.N.S., K.M.M., and A.T.T. wrote the manuscript and gave final approval of the manuscript for submission.
Conflict-of-interest disclosure: K.M.M. has been or is a consultant for Novartis, Celgene Corp (Bristol Myers Squibb), Agios Pharmaceuticals, CRISPR Therapeutics, Vifor Pharma, and Pharmacosmos; and received research funding from Agios Pharmaceuticals and Pharmacosmos. A.T.T. has been or is consultant for Novartis, Celgene Corp (Bristol Myers Squibb), Vifor Pharma, Silence Therapeutics, and Ionis Pharmaceuticals; and received research funding from Novartis, Celgene Corp (Bristol Myers Squibb), La Jolla Pharmaceutical Company, Roche, Protagonist Therapeutics, and Agios Pharmaceuticals. A.N.S. declares no competing financial interests.
Correspondence: Ali T. Taher, Department of Internal Medicine, American University of Beirut Medical Center, PO Box 11-0236, 1107 2020 Beirut, Lebanon; e-mail: ataher@aub.edu.lb.
References
Author notes
All data are available on request from the corresponding author, Ali T. Taher (ataher@aub.edu.lb).
The online version of this article contains a data supplement.