In this issue of Blood, Mei and colleagues report the results of a trial in which the histone deacetylase inhibitor vorinostat was combined with the programmed cell death protein-1 inhibitor (PD1i) pembrolizumab, demonstrating an impressive response rate, including in patients who were refractory to prior PD1i therapy.1
Classic Hodgkin lymphoma (cHL) is highly sensitive to treatment with inhibitors of PD1i, thought to be at least partly due to high expression of PD-L1, the ligand for PD1, by the malignant Hodgkin and Reed-Sternberg (HRS) cell. The genetic locus for PD-L1 is found on chromosome 9p24, which is itself subject to frequent copy number alterations.2 Initial phase 2 trials demonstrated high and durable response rates of single-agent nivolumab and pembrolizumab in relapsed or refractory cHL.3,4 Although some response are durable, most patients either fail to respond or lose their response at some point during therapy.
It was by no means obvious that PD1 inhibition would work in cHL as HRS cells frequently do not express both major histocompatibility complex (MHC) class I and β-2-microglobulin. This would make a CD8+ cytotoxic T-cell-mediated mechanism of action unlikely. Further work has instead focused on CD4+ T cells as these are often colocalized with HRS cells and PD-L1 expressing tumor-associated macrophages.5 Moreover, in a subset of patients who had relapsed more than 12 months after an autologous stem cell transplant, expression of MHC class II (the receptor for CD4) was associated with a longer progression-free survival.2 It is hypothesized that CD4+ effector T cells maybe at least one of the mediators of efficacy of PD1i in cHL. Further support comes from analysis of immune signatures in patients with cHL on PD inhibitor therapy. An expansion of CD4 (but not CD8) T-cell receptor clonal diversity was associated with response, most strikingly seen in those achieving a complete response.6
HDAC inhibitors also have demonstrable, albeit modest, activity in relapsed or refractory cHL with a large phase 2 study of panobinostat reporting a rather disappointing overall response rate of 27% and complete response rate of 4%.7 Significant interest in HDAC inhibitors in combination with immunotherapy agents like PD1is has arisen with reports of an immunostimulatory action. Epigenetic modifying agents have been shown in various models to enhance MHC class I expression, increase antigen presentation, and increase T-cell infiltration to the tumor microenvironment.8
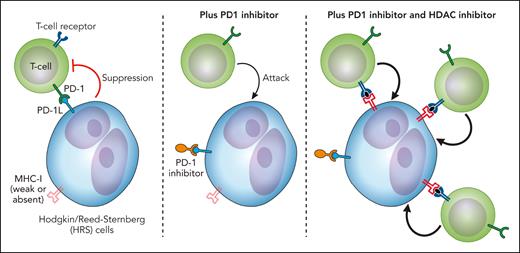
It can therefore be hypothesized that HDAC inhibition may lead to an improved or even restored sensitivity to PD1 inhibition in cHL. In this study reported by Mei and coworkers, 32 patients with relapsed or refractory disease were treated with vorinostat and pembrolizumab, 30 at the recommended phase 2 dose. Of note, almost all had received prior brentuximab vedotin therapy and 78% had received prior PD1i therapy with 56% being PD1i refractory. The best overall response rate was 72% with 56% of PD1i refractory patients responding and 11% having a complete metabolic response. The investigators secured pretreatment and postprogression biopsies in 3 patients. Although there was no relative difference in immune cell subsets, there was an increased density of CD4+ and CD8+ T cells in 2 out of 3 patients. In an attempt to investigate the potential mechanisms leading to immune cell expansion, Hodgkin lymphoma cell lines were treated with vorinostat and subjected to RNA sequencing. Upregulated genes included groups of genes involved in cytotoxic T-cell recruitment, T-cell costimulation, and antigen presentation. Although Hodgkin cell lines are not necessarily a true reflection of cHL in vivo, this lends support to a model such as that illustrated in the figure whereby HDAC inhibitors may sensitize some patients to PD1 inhibition. Such sensitization (or indeed resensitization) may include increased expression of immune receptors by HRS cells, increased recruitment, and enhanced activity of cytotoxic T cells.
A model showing how the addition of an HDAC inhibitor may enhance the activity of a PD1i in cHL through increased immune receptor expression (such as MHC class I), increased recruitment, and activation of T cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
A model showing how the addition of an HDAC inhibitor may enhance the activity of a PD1i in cHL through increased immune receptor expression (such as MHC class I), increased recruitment, and activation of T cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
Mei and colleagues have made an important contribution, showing proof of principle that PD1i activity may be enhanced by incorporating a rational, nonchemotherapy, targeted agent in combination. Although it is increasingly appreciated that PD1is combined with cytotoxic chemotherapy can lead to very high response rates, a desirable outcome in the field is to move away from cytotoxic agents and introduce targeted therapies predicted to synergize with PD1is. To this end a number of trials of drugs targeting multiple immune checkpoints are ongoing, including combined LAG3 and PD1 inhibition and combined TIM3 and PD1 blockade. Hodgkin lymphoma was one of the first cancers to be frequently cured by radiotherapy and then by combination chemotherapy. It will fascinating to see if immunotherapy combinations hold similar potential.
Conflict-of-interest disclosure: G.P.C. reports research funding received from Bristol Myers Squibb.