Abstract
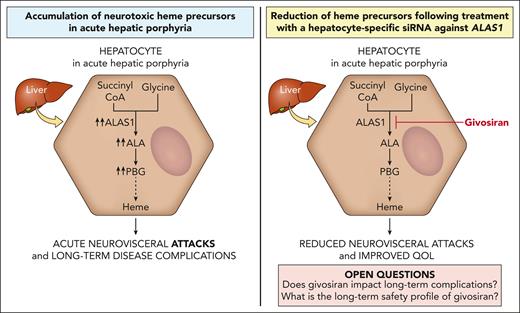
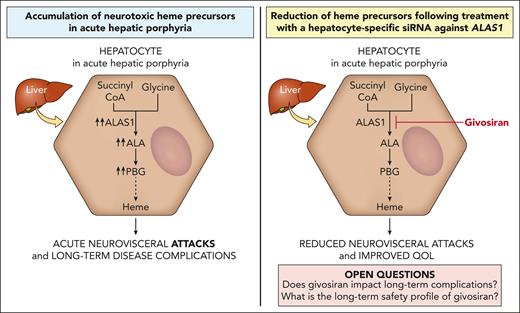
The acute hepatic porphyrias (AHPs) are inherited disorders of heme biosynthesis characterized by life-threatening acute neurovisceral attacks precipitated by factors that upregulate hepatic 5-aminolevulinic acid synthase 1 (ALAS1) activity. Induction of hepatic ALAS1 leads to the accumulation of porphyrin precursors, in particular 5-aminolevulinic acid (ALA), which is thought to be the neurotoxic mediator leading to acute attack symptoms such as severe abdominal pain and autonomic dysfunction. Patients may also develop debilitating chronic symptoms and long-term medical complications, including kidney disease and an increased risk of hepatocellular carcinoma. Exogenous heme is the historical treatment for attacks and exerts its therapeutic effect by inhibiting hepatic ALAS1 activity. The pathophysiology of acute attacks provided the rationale to develop an RNA interference therapeutic that suppresses hepatic ALAS1 expression. Givosiran is a subcutaneously administered N-acetylgalactosamine–conjugated small interfering RNA against ALAS1 that is taken up nearly exclusively by hepatocytes via the asialoglycoprotein receptor. Clinical trials established that the continuous suppression of hepatic ALAS1 mRNA via monthly givosiran administration effectively reduced urinary ALA and porphobilinogen levels and acute attack rates and improved quality of life. Common side effects include injection site reactions and increases in liver enzymes and creatinine. Givosiran was approved by the US Food and Drug Administration and European Medicines Agency in 2019 and 2020, respectively, for the treatment of patients with AHP. Although givosiran has the potential to decrease the risk of chronic complications, long-term data on the safety and effects of sustained ALAS1 suppression in patients with AHP are lacking.
AHPs pathophysiology, clinical presentation, and treatment
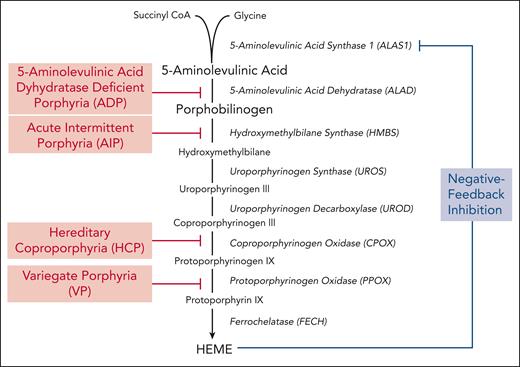
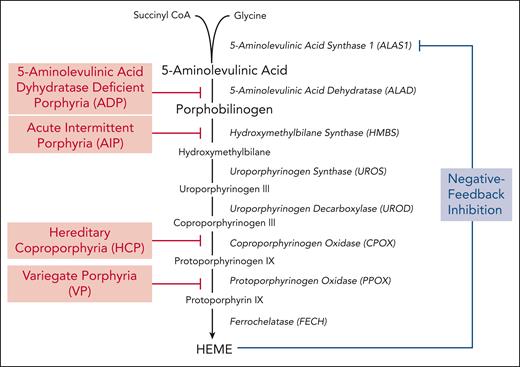
The porphyrias are a group of metabolic diseases, each caused by a defect in a specific enzyme in the heme biosynthetic pathway, resulting in the accumulation of pathway intermediates. Disease phenotype, whether neurologic and/or photocutaneous symptoms, is determined by the site and composition of heme intermediates that accumulate. Porphyrias are classified pathophysiologically as either hepatic or erythropoietic based on the primary site of overproduction and accumulation of heme pathway intermediates.1 The acute hepatic porphyrias (AHPs) include 3 autosomal dominant disorders (acute intermittent porphyria [AIP], hereditary coproporphyria, and variegate porphyria) and the ultrarare autosomal recessive disorder, 5-aminolevulinic acid dehydrogenase deficiency porphyria (ADP), although the relevant sites of heme intermediate overproduction in ADP remain unclear.2,3 The enzyme deficiencies in each AHP are shown in Figure 1.
Hepatic heme biosynthetic pathway and associated AHPs. Heme biosynthesis involves the conversion of glycine and succinyl CoA into heme via 8 enzymatic steps (enzymes are shown in italics). The first and rate-limiting enzyme, 5-aminolevulinic acid synthase 1 (ALAS1), uses pyridoxal 5-phosphate (or active vitamin B6) as a cofactor to condense glycine and succinyl CoA into 5-aminolevulinic acid (ALA). Two molecules of ALA are then condensed to form the monopyrrole porphobilinogen (PBG) through the action of the second enzyme, 5-aminolevulinic acid dehydratase. ALA and PBG are porphyrin precursors, and ALA in particular is believed to be neurotoxic. The third enzyme, hydroxymethylbilane synthase (HMBS), converts PBG to the linear tetrapyrrole, hydroxymethylbilane, which is then converted to the circular tetrapyrrole, uroporphyrinogen III, via uroporphyrinogen synthase. The subsequent 2 enzymatic reactions modify the side chains of the porphyrinogens, whereas the penultimate enzyme, protoporphyrinogen oxidase, oxidizes protoporphyrinogen IX to protoporphyrin IX. Finally, ferrochelatase inserts ferrous iron into protoporphyrin IX to form heme. In hepatocytes, heme synthesis is controlled by ALAS1 activity, which is regulated by the end-product, heme. Under heme-replete conditions, heme represses ALAS1 activity, whereas under heme-deficient conditions, this negative feedback inhibition is lost and heme synthesis is induced. The AHPs, shown in red, include 4 disorders that are caused by partial deficiencies of the corresponding heme biosynthetic enzymes.
Hepatic heme biosynthetic pathway and associated AHPs. Heme biosynthesis involves the conversion of glycine and succinyl CoA into heme via 8 enzymatic steps (enzymes are shown in italics). The first and rate-limiting enzyme, 5-aminolevulinic acid synthase 1 (ALAS1), uses pyridoxal 5-phosphate (or active vitamin B6) as a cofactor to condense glycine and succinyl CoA into 5-aminolevulinic acid (ALA). Two molecules of ALA are then condensed to form the monopyrrole porphobilinogen (PBG) through the action of the second enzyme, 5-aminolevulinic acid dehydratase. ALA and PBG are porphyrin precursors, and ALA in particular is believed to be neurotoxic. The third enzyme, hydroxymethylbilane synthase (HMBS), converts PBG to the linear tetrapyrrole, hydroxymethylbilane, which is then converted to the circular tetrapyrrole, uroporphyrinogen III, via uroporphyrinogen synthase. The subsequent 2 enzymatic reactions modify the side chains of the porphyrinogens, whereas the penultimate enzyme, protoporphyrinogen oxidase, oxidizes protoporphyrinogen IX to protoporphyrin IX. Finally, ferrochelatase inserts ferrous iron into protoporphyrin IX to form heme. In hepatocytes, heme synthesis is controlled by ALAS1 activity, which is regulated by the end-product, heme. Under heme-replete conditions, heme represses ALAS1 activity, whereas under heme-deficient conditions, this negative feedback inhibition is lost and heme synthesis is induced. The AHPs, shown in red, include 4 disorders that are caused by partial deficiencies of the corresponding heme biosynthetic enzymes.
Patients with AHP present with episodic attacks of severe abdominal pain and acute autonomic and other neurologic dysfunction (eg, seizures and posterior reversible encephalopathy).4 Most of the symptomatic patients are female, with disease onset after puberty.5-7 Various factors precipitate the acute attacks by increasing the expression of hepatic 5-aminolevulinic acid synthase 1 (ALAS1), either by directly promoting its transcription (eg, dieting or exogenous/endogenous progesterone8-11) and/or by consuming heme and increasing heme demand in the liver12 (eg, cytochrome p450-inducing drugs or exogenous/endogenous progesterone13,14). When ALAS1 activity is markedly increased, the respective enzyme deficiencies create a metabolic bottleneck, leading to insufficient hepatic heme production and in turn loss of the negative feedback inhibition of heme on ALAS1.15 Consequently, ALAS1 activity is further upregulated, and the porphyrin precursors, ALA and porphobilinogen (PBG), accumulate in the liver. Several hypotheses have been proposed to explain the neurologic dysfunction in the AHPs.12 Porphyrin precursors, ALA in particular, are likely neurotoxic,16-18 and their hepatic accumulation19,20 is thought to be a major mediator of neurotoxicity in acute attacks. Symptomatic improvement after an acute attack correlates with decreased ALA levels.21 Some patients demonstrate persistent elevations in ALA and PBG after an attack. The pathophysiologic implication of this finding to the neurologic complications and why some patients develop chronic neurologic symptoms remain open questions. In variegate porphyria and hereditary coproporphyria, porphyrins (in addition to porphyrin precursors) accumulate, which are photosensitizers and account for the cutaneous photosensitivity of these 2 AHP subtypes.
Disease penetrance in the AHPs is low, with most of the genetically diagnosed patients never experiencing an acute attack. AIP, the most common AHP, is caused by heterozygous pathogenic variants in hydroxymethylbilane synthase (HMBS), and the prevalence of pathogenic HMBS variants is ∼1/1700 Caucasians22,23 while the estimated disease penetrance is ∼1% in the general population. The disease penetrance is notably higher, ∼20% to 40% in families with symptomatic AIP heterozygotes, suggesting that genetic modifiers and/or environmental factors modulate susceptibility to acute attacks.23-25 Most symptomatic patients have only a few attacks in their lifetime, however ∼5% of symptomatic patients with AIP experience recurrent attacks.26,27 Importantly, patients with AIP with recurrent attacks (≥4 attacks/year) commonly report chronic symptoms between attacks.5,28 A contemporary natural history study of mostly patients with AIP with recurrent attacks found that ≥80% of patients reported chronic symptoms when not having an attack (eg, pain, mood/sleep disturbances, gastrointestinal/bladder symptoms) and high healthcare use.29,30 Hepatic ALAS1 mRNA and ALA and PBG levels often remain elevated between attacks, particularly in AIP, suggesting that a component of the chronic symptoms suffered by patients may reflect active disease.30 Moreover, AHPs are associated with long-term coexisting illnesses, including chronic kidney disease, hypertension, chronic neuropathy, and liver disease, which may manifest as elevated aminotransferase levels, fibrosis, cirrhosis, and/or hepatocellular carcinoma.5,31
Treatment of an AHP attack includes addressing symptoms and complications of the disease (eg, pain management), eliminating known triggers that increase hepatic ALAS1 activity and precipitate or worsen an attack, and intravenous carbohydrate (glucose) and hemin loading.32,33 Hemin (heme arginate in Europe and lyophilized hematin in the United States) is thought to act by repressing hepatic ALAS1 activity and hence decreasing the overproduction of ALA and PBG. Treatment reduces urinary ALA and PBG levels and improves symptoms in most patients after 3 to 4 daily infusions.34-40 Common side effects of hemin include infusion-site phlebitis, partially ameliorated by reconstituting the drug in albumin rather than sterile water,41 and iron overload after multiple treatments42 (100 mg of hemin contains ∼8 mg of elemental iron43).
Management of patients suffering from recurrent attacks is challenging. Case series support some benefit from off-label prophylactic or “on demand” infusions of hemin in the outpatient setting given at various dosing frequencies; however, some patients continue to suffer attacks and develop chronic pain.5,39,44 Moreover, this approach often requires central venous access, which itself carries risk. Recurrent attacks during the luteal phase of the menstrual cycle have been treated with gonadotropin-releasing hormone analogs to suppress ovulation, with a mixed response45,46 and at the expense of potential bone mineral density loss and postmenopausal symptoms. Liver transplantation is a last-resort treatment option for select patients with recurrent attacks that are refractory to treatment and lead to poor quality of life, in whom the benefit is felt to outweigh its associated risks of morbidity and mortality.47-49 RNA interference (RNAi; givosiran) against ALAS1 offers another option for these patients and is the focus of this review and the only US Food and Drug Administration (FDA)–approved preventative therapy currently available for the AHPs. Its therapeutic benefit in treating ongoing acute attacks in patients remains unexplored.
Preclinical studies to develop an effective RNAi therapeutic targeting hepatic Alas1
The central role of hepatic ALAS1 induction causing elevated ALA and PBG levels in the pathogenesis of the acute attacks provided the rationale to develop a RNAi therapeutic targeting hepatic ALAS1 for the prevention and treatment of acute attacks in AHPs. RNAi therapeutics leverage a naturally occurring regulatory process in which double-stranded RNAs are cleaved into short fragments known as short interfering RNAs (siRNAs), which subsequently bind to mRNAs in a sequence-specific manner and trigger their degradation.50
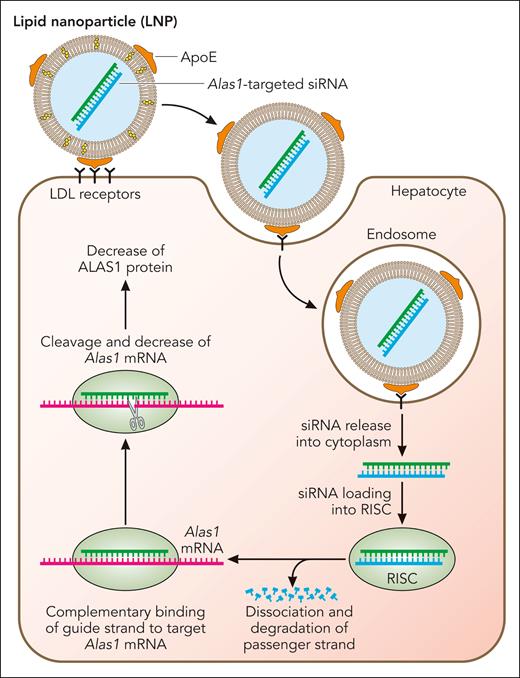
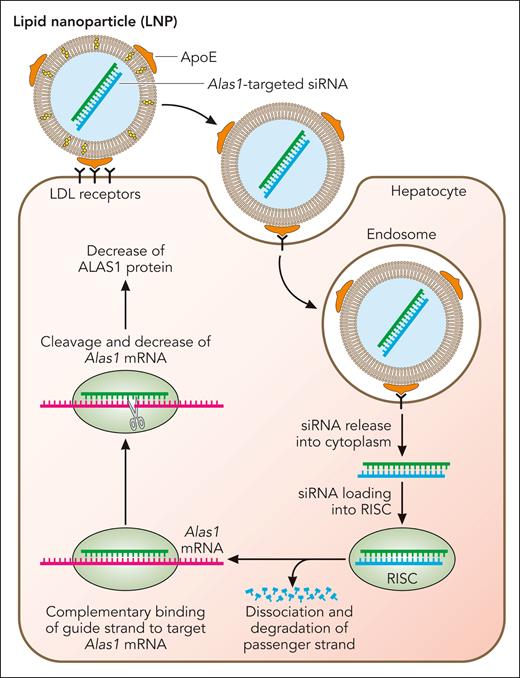
To facilitate preclinical studies, a highly potent siRNA duplex specific for the murine Alas1 mRNA (Alas1-siRNA) was identified and encapsulated in proprietary biodegradable lipid nanoparticles (LNPs) for efficient hepatic delivery following intravenous administration.51 As shown in Figure 2, the LNPs consist of ionizable cationic lipids that are neutral at physiologic pH and acquire apolipoprotein E on their surface while in circulation, which targets them for uptake by low-density lipoprotein receptors that are primarily expressed on hepatocytes52-54 (Figure 2). After clathrin-mediated endocytosis and acidification of the endocytic vesicle, the ionizable lipids on the surfaces of the LNP become positively charged and interact with anionic phospholipids in the endosomal membrane, leading to LNP-endosomal membrane fusion.55 Subsequently, Alas1-siRNA is released into the hepatocyte cytoplasm and is loaded into the RNA-induced silencing complex (RISC), where the double strands unwind and the guide strand loads onto the RISC complex while the passenger strand gets degraded (Figure 2). The guide strand-loaded RISC complex binds Alas1 mRNA in a complementary manner and degrades the target mRNA, ultimately leading to decreased ALAS1 protein expression.
Mechanism of LNP-mediated Alas1 silencing in hepatocytes. The LNPs encapsulating the double-stranded Alas1-siRNA consist of cationic, ionizable lipids that are positively charged at acidic pH but neutral at physiologic pH. While in circulation, the LNPs acquire apolipoprotein E on their surface, which is bound primarily by low-density lipoprotein receptors that are highly expressed on the surface of hepatocytes. This induces clathrin-mediated endocytosis and the formation of early endosomes. As the endosomes mature and the pH becomes highly acidic, the ionizable lipids turn positive and bind to the negative lipids that are exposed on the endosomal membrane, leading to membrane fusion and disruption of the LNP. This allows escape of the Alas1-siRNA into the hepatocyte cytoplasm, where it is loaded onto the RISC. The passenger (sense) strand is degraded, leaving only the guide (antisense) strand on the RISC, which binds endogenous Alas1 mRNA in a complementary manner and degrades it. Professional illustration by Patrick Lane, ScEYEnce Studios.
Mechanism of LNP-mediated Alas1 silencing in hepatocytes. The LNPs encapsulating the double-stranded Alas1-siRNA consist of cationic, ionizable lipids that are positively charged at acidic pH but neutral at physiologic pH. While in circulation, the LNPs acquire apolipoprotein E on their surface, which is bound primarily by low-density lipoprotein receptors that are highly expressed on the surface of hepatocytes. This induces clathrin-mediated endocytosis and the formation of early endosomes. As the endosomes mature and the pH becomes highly acidic, the ionizable lipids turn positive and bind to the negative lipids that are exposed on the endosomal membrane, leading to membrane fusion and disruption of the LNP. This allows escape of the Alas1-siRNA into the hepatocyte cytoplasm, where it is loaded onto the RISC. The passenger (sense) strand is degraded, leaving only the guide (antisense) strand on the RISC, which binds endogenous Alas1 mRNA in a complementary manner and degrades it. Professional illustration by Patrick Lane, ScEYEnce Studios.
Proof of concept of this RNAi therapeutic was established using a murine model of AIP that has ∼30% of normal HMBS enzymatic activity. Treatment of these mice with serial injections of phenobarbital, a prototypic porphyrinogenic agent, induces hepatic Alas1 expression and leads to massive accumulation of plasma and urinary ALA and PBG, akin to a clinical acute attack in patients.56
When AIP mice were administered a single infusion of the lead LNP-encapsulated Alas1-siRNA (LNP-Alas1) and then challenged with serial phenobarbital injections, hepatic Alas1 and plasma and urinary ALA and PBG remained at baseline levels, demonstrating that LNP-Alas1 completely prevented the acute attack.51 LNP-Alas1 was also highly effective in treating an ongoing acute attack, as a single infusion into AIP mice pretreated with phenobarbital rapidly decreased the elevated plasma ALA and PBG concentrations. Importantly, hepatic heme content appeared unaltered by a single therapeutic dose of LNP-Alas1, as heme saturation levels or activities of representative hepatic hemoproteins, tryptophan dioxygenase and CYP2E1, were maintained.51
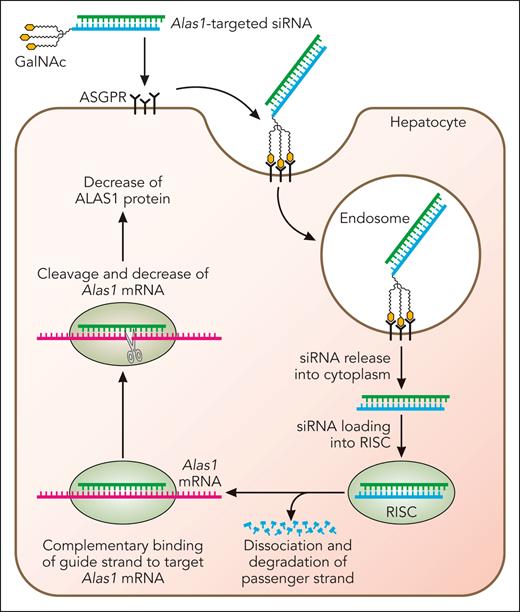
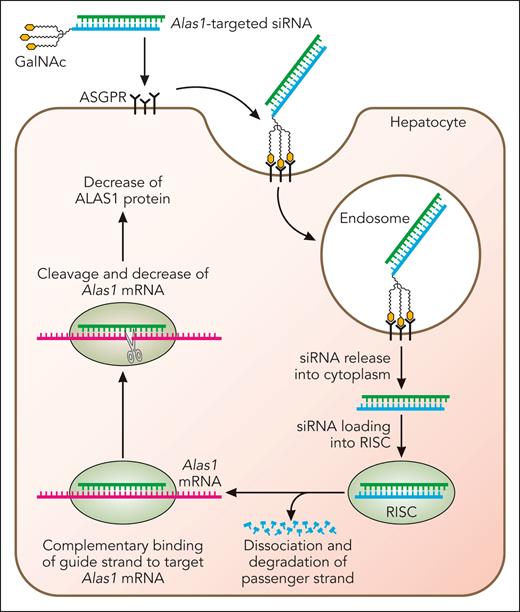
Subsequent efforts were directed to develop an Alas1-siRNA conjugated to trivalent N-acetyl galactosamine (GalNAc), as this approach permits subcutaneous administration. As depicted in Figure 3, the GalNAc-conjugated Alas1-siRNA (GalNAc-Alas1) is recognized and bound by asialoglycoprotein receptors that are abundantly and nearly exclusively expressed on hepatocytes. This induces clathrin-mediated endocytosis and transport of the GalNAc-Alas1 to the endosomal compartment, where its acidic pH causes the siRNA to be released from asialoglycoprotein receptors, which are then rapidly recycled to the hepatocyte cell surface. Alas1-targeted siRNA is released into the cytosol, loaded onto the RISC complex, and thereafter, represses Alas1 mRNA expression (Figure 3).
Mechanism of GalNAc-conjugated siRNA-mediated silencing of hepatocyte Alas1. Alas1-siRNA is conjugated to trivalent GalNAc, which is recognized and bound by asialoglycoprotein receptors that are abundantly and near-exclusively expressed on the surface of hepatocytes. This induces clathrin-mediated endocytosis and the formation of early endosomes, which mature into late endosomes that are highly acidic. The lower pH results in the release of the Alas1-siRNA from the asialoglycoprotein receptors, which are then rapidly recycled to the hepatocyte cell surface. Alas1-siRNAs then escape to the hepatocyte cytoplasm and are loaded onto RISC. The passenger (sense) strand is degraded, leaving only the guide (antisense) strand on the RISC, which binds endogenous Alas1 mRNA in a complementary manner and degrades it. Professional illustration by Patrick Lane, ScEYEnce Studios.
Mechanism of GalNAc-conjugated siRNA-mediated silencing of hepatocyte Alas1. Alas1-siRNA is conjugated to trivalent GalNAc, which is recognized and bound by asialoglycoprotein receptors that are abundantly and near-exclusively expressed on the surface of hepatocytes. This induces clathrin-mediated endocytosis and the formation of early endosomes, which mature into late endosomes that are highly acidic. The lower pH results in the release of the Alas1-siRNA from the asialoglycoprotein receptors, which are then rapidly recycled to the hepatocyte cell surface. Alas1-siRNAs then escape to the hepatocyte cytoplasm and are loaded onto RISC. The passenger (sense) strand is degraded, leaving only the guide (antisense) strand on the RISC, which binds endogenous Alas1 mRNA in a complementary manner and degrades it. Professional illustration by Patrick Lane, ScEYEnce Studios.
GalNAc-Alas1 also effectively prevented acute attacks in the AIP mice when administered subcutaneously once a week for 4 consecutive weeks (Figure 4). Remarkably, these 4 doses provided significant protection against the induction of an acute attack for at least 6 weeks (Figure 4). The long-lasting activity of GalNAc-conjugated siRNAs has been attributed to their stability in highly acidic endosomal compartments, which serve as long-term depots for the siRNA.57 Notably, a single GalNAc-Alas1 dose was highly effective in treating an ongoing attack, as it drove down the elevated plasma ALA and PBG concentrations much more rapidly and effectively compared with 2 consecutive daily intravenous doses of hemin (4.0 mg/kg per day) (Figure 5). Based on these promising results, a GalNAc-conjugated siRNA targeting the human ALAS1 sequence (givosiran) was developed and evaluated for its safety in rodents and nonhuman primates before advancing to clinical trials.
GalNAc-Alas1 effectively prevents phenobarbital-induced attacks in AIP mice. AIP mice were subcutaneously administered GalNAc-Alas1 (denoted by black triangles [▼]; 3 mg/kg per dose) once a week for 4 consecutive weeks and then challenged with serial phenobarbital injections (denoted by arrows [↓]; doses were 110, 120, and 130 mg/kg per day) at weeks (W) 0, 2, 4, and 6 after the last siRNA dose to determine the effectiveness and durability of GalNAc-Alas1 to prevent attacks. Blood samples were collected (depicted by X marks), and plasma ALA and PBG concentrations were determined. Data shown are means + standard deviations. ∗P < .01 and ∗∗P < .005 vs control AIP mice that were phenobarbital-induced after treatment with saline (no GalNAc-Alas1), t-test (n = 3-4).
GalNAc-Alas1 effectively prevents phenobarbital-induced attacks in AIP mice. AIP mice were subcutaneously administered GalNAc-Alas1 (denoted by black triangles [▼]; 3 mg/kg per dose) once a week for 4 consecutive weeks and then challenged with serial phenobarbital injections (denoted by arrows [↓]; doses were 110, 120, and 130 mg/kg per day) at weeks (W) 0, 2, 4, and 6 after the last siRNA dose to determine the effectiveness and durability of GalNAc-Alas1 to prevent attacks. Blood samples were collected (depicted by X marks), and plasma ALA and PBG concentrations were determined. Data shown are means + standard deviations. ∗P < .01 and ∗∗P < .005 vs control AIP mice that were phenobarbital-induced after treatment with saline (no GalNAc-Alas1), t-test (n = 3-4).
GalNAc-Alas1 effectively treats ongoing induced attacks in AIP mice. AIP mice were induced with a combination of serial phenobarbital injections and diethoxycarbonyl-1,4-dihydrocollidine (DDC) (denoted by arrows [↓]; phenobarbital doses were 90, 100, 110, and 90 mg/kg per day, whereas DDC dose was 20 mg/kg per day) for 4 consecutive days to markedly elevate plasma ALA and PBG concentrations and treated with either a single subcutaneous dose of GalNAc-Alas1 (denoted by a black triangle [▼]; 3 mg/kg), 2 daily infusions of hemin (represented by white triangles [▽]; 4 mg/kg), or saline (controls), as shown. Blood samples were collected (represented by X marks) at the indicated times, and plasma ALA and PBG concentrations were determined. Data shown are means + standard deviations (n = 3-5).
GalNAc-Alas1 effectively treats ongoing induced attacks in AIP mice. AIP mice were induced with a combination of serial phenobarbital injections and diethoxycarbonyl-1,4-dihydrocollidine (DDC) (denoted by arrows [↓]; phenobarbital doses were 90, 100, 110, and 90 mg/kg per day, whereas DDC dose was 20 mg/kg per day) for 4 consecutive days to markedly elevate plasma ALA and PBG concentrations and treated with either a single subcutaneous dose of GalNAc-Alas1 (denoted by a black triangle [▼]; 3 mg/kg), 2 daily infusions of hemin (represented by white triangles [▽]; 4 mg/kg), or saline (controls), as shown. Blood samples were collected (represented by X marks) at the indicated times, and plasma ALA and PBG concentrations were determined. Data shown are means + standard deviations (n = 3-5).
Because ALAS1 is a nonsecretory protein, one challenge in monitoring the therapeutic effectiveness of the Alas1-siRNA in patients was the inability to directly assess ALAS1 mRNA levels without serial liver biopsies. To overcome this challenge, a circulating extracellular mRNA detection (cERD) assay that measures Alas1 mRNA expression levels in serum and urinary exosomes was developed.58 This was based on previous studies showing that hepatocytes secrete specific mRNAs in exosomes, which are then released into the serum or urine and reflect levels of mRNA expression in the liver.59,60 Indeed, serum and urinary exosome Alas1 mRNA expression in AIP mice faithfully reflected those in the liver, both after phenobarbital induction and GalNAc-Alas1 treatment.58 This cERD assay permits assessment of basal hepatic ALAS1 mRNA status in patients with AHPs and related disorders,2,4 and importantly, allows for noninvasive monitoring of hepatic ALAS1 mRNA levels after RNAi-mediated silencing.
Clinical studies on the effectiveness of hepatocyte-targeted GalNAc-conjugated ALAS1-siRNA (givosiran)
Clinical studies established the effectiveness of givosiran to continuously suppress hepatic ALAS1 and urinary ALA and PBG levels and to reduce acute attack rates in the AHPs. A multicenter, randomized, placebo-controlled phase 1 trial of givosiran for AHP was conducted in 3 parts (supplemental Table 1, available on Blood website).61 In parts A and B, single- and multiple-dose cohort studies in asymptomatic patients with AHP with elevated urinary ALA and PBG levels (so-called “chronic high excretors”) demonstrated dose-dependent reductions from baseline in urinary exosome ALAS1 mRNA, ALA, and PBG levels, which were sustained up to day 70. Urinary exosome ALAS1 mRNA was measured using the aforementioned cERD assay as a surrogate for hepatic ALAS1 mRNA.58 As expected, urinary exosome ALAS1 mRNA and urinary ALA measurements correlated closely with one another. Part C was conducted in patients with recurrent attacks, defined as ≥2 attacks in the previous 6 months or on prophylactic hemin therapy. Patients were randomized (3:1) to receive givosiran or placebo dosed quarterly or monthly. Urinary exosome ALAS1 mRNA decreased at all dosing levels, although monthly dosing led to more sustained reductions in ALAS1 mRNA and urinary ALA and PBG levels compared with quarterly dosing. This corresponded clinically to a reduction in the mean annualized attack rate in the givosiran-treated group compared with the placebo (7.2 vs 16.7) and a 48% reduction in annualized hemin usage. Antidrug antibodies were rare and did not affect safety or efficacy.62,63 The most common adverse events were injection site reactions, abdominal pain, nausea, diarrhea, and nasopharyngitis.
A phase 3, randomized, double-blind, placebo-controlled trial of 94 patients with AHP with recurrent attacks (ENVISION study) randomly assigned patients 1:1 to receive placebo or givosiran (2.5 mg/kg subcutaneously monthly) (supplemental Table 1).63 Patients with AIP who were treated with givosiran showed a 74% reduction (P < .001) in the mean annualized attack rate, a reduction in hemin usage, and improved quality of life as measured by the patient global impression of change questionnaire compared with patients receiving placebo. Fatigue, nausea, decreased estimated GFR (eGFR), and increased creatinine and alanine transaminase (ALT) were more common in patients treated with givosiran compared with patients receiving placebo. A decline in kidney function was seen in 5 patients in the givosiran-treated group. Renal biopsies in 2 of these patients showed underlying porphyria-related kidney disease. ALT elevations greater than threefold the upper limit of normal occurred in 14.6% of patients treated with givosiran compared with 2.2% of patients receiving placebo. The drug was discontinued per protocol in one patient who developed an ALT elevation greater than 8 times the upper limit of normal.
A 24-month efficacy analysis showed sustained reductions in urinary ALA and PBG levels and in the annualized attack rate in givosiran-treated patients and a significant decrease in the annualized attack rate in patients who crossed over from placebo to active drug.64 Sustained reductions in urinary ALA and PBG levels are associated with decreases in hemin use, pain, opioid use, and improved quality of life. Hepatic adverse events characterized primarily as elevations in ALT were seen in 18% of patients treated with givosiran and were mild to moderate in severity. These transaminase elevations typically occurred within the first 3 to 6 months of treatment and resolved with ongoing treatment. Twenty-two percent of patients treated with givosiran had a decline in eGFR and an increase in creatinine, which were not progressive.64 It was speculated that givosiran may accumulate in kidney tubules and lead to decreased Alas1 in animal models.65 However, preclinical studies with high doses of givosiran in rodents showed no effect of Alas1 mRNA expression in the kidney.66
Clinical experience with givosiran
Based on the positive clinical trial data, givosiran (Givlaari) was approved by the FDA in November 2019 for adults with AHP at a dose of 2.5 mg/kg subcutaneously monthly.67 It was approved by the European Medicines Agency in 2020 for patients 12 years of age and older. Listed precautions include monitoring for anaphylaxis, injection site reactions, and hepatic and renal toxicity. Givosiran is not recommended for women who are pregnant or breastfeeding because of limited safety information. Data collection through a global registry is ongoing to characterize the natural history and management of patients with AHP and to further characterize the real-world safety and efficacy of givosiran.68
Clinical experience in France suggests that the biologic activity of givosiran, reflected in the median reduction in urinary ALA levels from baseline, is highest in patients treated earlier in their disease course.69 Notably, in a subset of patients reported in this small cohort study, givosiran dosing frequency was adapted (given less frequently than monthly) based on monthly measurements of urinary ALA levels without compromising treatment efficacy. Some patients had prolonged and stably low ALA levels without recurrent givosiran treatment. Based on this experience, the authors suggested the potential for personalizing treatment approaches in the future with this therapeutic.69
Real-world clinical experience demonstrates a transient decrease in kidney function in most patients treated with givosiran with no signs of drug-induced injury.70 The decline in renal function was higher in patients with lower baseline eGFR. Renal biopsy of 1 patient showed normal tubular ALAS1 expression. The authors hypothesize that alterations in intrarenal hemodynamics may be a contributing factor. Given the available data, close monitoring of kidney function is indicated in patients treated with givosiran. Clinical experience from France reported ALT elevations in 32% of their patients treated with givosiran.69 Treatment was interrupted only if the ALT measured >5× the upper limit of normal. Among their patients with ALT elevations, all had resolution of their ALT elevations with continued dosing.
Several case series have reported elevated plasma homocysteine levels in patients with AHP receiving givosiran.71-74 Hyperhomocysteinemia has also been reported in patients with AHP in the absence of givosiran exposure.75,76 Vitamin B6 supplementation has been reported to reduce or normalize homocysteine levels in both patients with AHP receiving givosiran and those not receiving givosiran.71,73 The precise mechanism underlying the hyperhomocysteinemia remains unknown.
Open questions on givosiran treatment in the AHPs
Although givosiran provides significant therapeutic benefit to patients with AHP, several relevant questions surrounding this therapeutic approach remain.
Are there negative consequences of sustained ALAS1 suppression?
By reducing hepatic ALAS1 activity, givosiran might theoretically reduce hepatic heme content and consequently the activity of heme-dependent hepatic proteins, such as the cytochrome p450 enzymes. This could, in turn, decrease the metabolism of concurrently administered medications. In a single drug-drug interaction study of 9 patients with biochemical evidence of AIP who remained asymptomatic, givosiran moderately inhibited CYP1A2 and CYP2D6, weakly inhibited CYP3A4 and CYP2C19, and had no effect on CYP2C9.77 CYP2D6 catalyzes the metabolism of a number of medications, including several antidepressants and neuroleptics. Some patients with AHP are treated with multiple medications to address the signs and symptoms of their disease. Whether clinically relevant drug-drug interactions will emerge on an individual basis in this polypharmacy setting remains a concern, so caution is advised in patients who require concomitant CYP1A2 or CYP2D6 substrates.
Why are plasma homocysteine levels elevated in patients with AHP, how does givosiran affect homocysteine metabolism, and what is the clinical significance of this hyperhomocysteinemia?
The observation of a co-increase in plasma homocysteine and methionine levels72,73 has led to the hypothesis that hyperhomocysteinemia in the AHPs, potentially exacerbated by givosiran, is because of hepatocyte heme deficiency leading to reduced function of cystathionine beta-synthase (CBS).74 CBS is a key homocysteine-metabolizing enzyme that requires heme for its full enzymatic activity. The finding of normal or minimally decreased exosomal ALAS1 mRNA levels in patients treated with givosiran suggests that hepatocytes are not heme deficient. The activities of CBS and cystathionine gamma lyase, another homocysteine-metabolizing enzyme, are also dependent on vitamin B6 as a cofactor.78 Vitamin B6 deficiency is reported in patients with AHP,76,79 perhaps reflecting the chronic hyperactivity of hepatic 5-aminolevulinic acid synthase, which also requires vitamin B6 (as a cofactor) or a nutritional deficiency of vitamin B6. Further investigation is required to understand homocysteine metabolism in the AHPs and how givosiran affects this. The clinical consequences of hyperhomocysteinemia in AHPs and the utility of monitoring plasma homocysteine and vitamin supplementation (ie, B vitamins and folate) are areas open for investigation.
Will givosiran affect the long-term complications of chronic pain and chronic kidney and liver disease in the AHPs?
In patients with AHP, chronic pain and chronic kidney and liver disease are linked to elevations in ALA and PBG levels.5,31,48,80 In the ENVISION trial, givosiran therapy reduced pain during attack-free periods compared with placebo,63 suggesting that a component of the chronic pain suffered by patients may be responsive to targeting ALAS1 activity. Recent studies have implicated ALA in the tubular injury in porphyria-associated kidney disease.31,81 ALA is freely filtered by the kidneys and reabsorbed in the proximal tubule by human peptide transporter 2.82 Patients with AHP harboring variants in peptide transporter 2 that result in increased renal ALA reabsorption are likely to have more severe chronic kidney disease compared with those with variants with decreased affinity for ALA.81 Any renal benefit of givosiran will have to outweigh the risk. Worsening chronic kidney disease after long-term givosiran therapy was reported in several patients with AHP with preexisting chronic kidney disease.70 An interesting feature of the hepatocellular carcinoma observed in patients with AHP, in contrast to non-AHP patients, is that it often occurs in the absence of a background of fibrosis or cirrhosis, suggesting a unique tumorigenesis. An informative study of 4 hepatocellular carcinomas developing in 3 patients with AIP demonstrated somatic mutations in the second HMBS allele in all 4 tumors and PBG (and presumably ALA) accumulation in the tumors.83 More broadly, ALA has been shown to induce oxidative stress and DNA damage in vitro.84,85 Collectively, these data suggest that long-term suppression of hepatic ALAS1 and the resulting normalization of ALA (and PBG) may prevent or ameliorate some of the chronic complications in AHP. Additional studies are needed to confirm this possibility.
Is there a role for givosiran in the treatment of acute attacks in patients with AHP?
Givosiran is FDA-approved for the prevention of acute attacks in AHP. Although murine studies support a role for givosiran in the treatment of acute attacks,51 this remains untested in patients. Givosiran treatment results in a rapid and dose-dependent reduction in urinary ALA and PBG toward the upper limit of normal in patients with AHP within hours of dosing,62 suggesting it is likely to be efficacious in acute attacks. Given the lack of clinical studies, acute attacks should be treated as per standard of care.
Can givosiran be safely administered to pregnant individuals?
There are no data on the use of givosiran in pregnant persons to evaluate its risk to the pregnancy and fetus, nor on whether the drug is present in human breast milk. Females of childbearing age should be counseled on this lack of data and provided contraception options before prescribing givosiran. In females who are planning pregnancy and who are medically stable, givosiran can be discontinued before pregnancy planning with close monitoring of symptoms. Hemin therapy has been given safely to pregnant women and could be considered.
Emerging therapies under development for AIP
Several alternative therapeutic approaches aimed at restoring deficient HMBS enzymatic activity in the liver are currently being developed for AIP, including mRNA-based gene therapy, enzyme replacement, and molecular chaperone therapy approaches. To date, clinical trials establishing efficacy in human disease are lacking for these therapies.
Initial gene therapy efforts using liver-targeted adeno-associated virus (AAV) to deliver a normal copy of the human HMBS (hHMBS) cDNA into AIP mice completely prevented the induction of acute biochemical attacks.86,87 However, phase I/II clinical trials in human patients with AIP failed to show clinical and/or biochemical benefit (ie, lowering of urinary ALA and PBG), presumably because of insufficient hepatocyte transduction.88 Therefore, subsequent efforts were directed to improve transgene expression by optimizing the promoter/enhancer of the therapeutic vector or by introducing sequence variants within the hHMBS cDNA that enhance HMBS activity.89,90
More recently, the effectiveness of LNP-mediated delivery of a mRNA encoding wild-type hHMBS (LNP-HMBS-mRNA) was evaluated in AIP mice.91 Intravenous administration of a single dose of LNP-HMBS mRNA completely prevented the induction of a phenobarbital-precipitated acute attack, and its protective effects were not diminished on repeated dosing. LNP-HMBS mRNA also rapidly and effectively decreased urinary ALA and PBG levels that were markedly elevated during a phenobarbital-induced attack. Treatment with LNP-HMBS mRNA prevented phenobarbital-induced systolic hypertension and mitochondrial insufficiency, which was previously shown to be present in AIP mouse livers, brains, and muscles.91-93
Although early efforts to replace the deficient HMBS enzyme using recombinant hHMBS enzyme were not successful in phase I/II clinical trials, recent preclinical studies have shown that conjugation of the hHMBS enzyme to apolipoprotein A1 (ApoA1), the major protein component of high-density lipoprotein that mediates its uptake by hepatocytes, prevents against phenobarbital-induced acute attacks when administered intravenously or subcutaneously into AIP mice.94
An alternative therapeutic approach that is still in the early preclinical stages is stabilizing the normal HMBS enzyme that is encoded by the nonmutated wild-type HMBS allele using allosteric molecular chaperones. Preclinical studies in the AIP mice showed modest increases (two- to threefold) of hepatic HMBS activity and incomplete protection against phenobarbital-induced acute attacks.95 Current efforts are directed to design more potent molecular chaperone candidates.
Concluding remarks
In the AHPs, RNAi technology provided the unique opportunity to suppress hepatocyte ALAS1 mRNA with high specificity. Givosiran is highly effective in reducing recurrent acute attacks and ameliorating chronic symptoms in patients with AHP. As of the end of 2022, 520 patients worldwide were receiving commercial drug.96 RNA therapeutics provide opportunities for the future development of AHP treatments, as exemplified by the mRNA-based gene therapy currently being developed for AIP. Additional long-term follow-up data are needed to fully understand the impact and safety of these therapies.
Acknowledgments
The Porphyrias Consortium (PC) is part of the Rare Diseases Clinical Research Network, which is funded by the National Institutes of Health (NIH) and led by the NIH National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation. PC is funded under grant number U54DK083909 as a collaboration between NIH NCATS and the NIH National Institute of Diabetes and Digestive and Kidney Diseases.
Authorship
Contribution: M.Y., S.K., and M.B. drafted and edited the manuscript and approved the submission.
Conflict-of-interest disclosure: M.Y. is a named inventor of intellectual property titled “siRNA-mediated therapy for the AHPs,” filed through the Icahn School of Medicine at Mount Sinai (ISMMS) and licensed to Alnylam Pharmaceuticals; as a coinventor, is entitled to a portion of the payments received by ISMMS; and also serves as a consultant for Alnylam Japan. S.K. is on an advisory board for Disc Medicine and has consulted for Alnylam Pharmaceuticals. M.B. has participated in advisory boards for Alnylam Pharmaceuticals, Recordati Rare Diseases, Disc Medicine, and Mitsubishi-Tanabe, and is a member of the scientific advisory board for the ELEVATE registry for AHPs sponsored by Alnylam Pharmaceuticals.
Correspondence: Manisha Balwani, Division of Medical Genetics and Genomics, Department of Genetics and Genomic Sciences and Medicine, Icahn School of Medicine at Mount Sinai, 1428 Madison Ave, 1st floor, New York, NY 10029; e-mail: manisha.balwani@mssm.edu.
References
Author notes
∗M.Y. and S.K. are joint first authors.
The online version of this article contains a data supplement.

![GalNAc-Alas1 effectively prevents phenobarbital-induced attacks in AIP mice. AIP mice were subcutaneously administered GalNAc-Alas1 (denoted by black triangles [▼]; 3 mg/kg per dose) once a week for 4 consecutive weeks and then challenged with serial phenobarbital injections (denoted by arrows [↓]; doses were 110, 120, and 130 mg/kg per day) at weeks (W) 0, 2, 4, and 6 after the last siRNA dose to determine the effectiveness and durability of GalNAc-Alas1 to prevent attacks. Blood samples were collected (depicted by X marks), and plasma ALA and PBG concentrations were determined. Data shown are means + standard deviations. ∗P < .01 and ∗∗P < .005 vs control AIP mice that were phenobarbital-induced after treatment with saline (no GalNAc-Alas1), t-test (n = 3-4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/19/10.1182_blood.2022018662/3/m_blood_bld-2022-018662-c-gr4.jpeg?Expires=1770932936&Signature=Sm0f0icr~z1a1tPbTp8kKXxx52V8R8znMTDuS~y6Ksz5kfJsgng9VvMnGp8PIIPh8FxXwBzTvuQrRfHEmKQo~NJdQVJ3n40oUVELW674BlY2Xb3vfBMoT5~CSi5mEhm5ggBXvaKTf7v8GvtOVJdbrU8A8Ki9JfkF20LBzirjDWhDeAKBVY-V3E61QAYrhIXfrHYAEllLrKPq18cIH4x~sfu4tTvSPD5816glacsIXvLtOSQDnfc2hd4MXg21YAgwX56XzaKp6hDbY0tlrowoOdDsdm7gQBpViQQylnH2zRrb~vr6r80IUr9ZgY2M8SEXyRqmytSZUFwxA6NwJh3jkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![GalNAc-Alas1 effectively treats ongoing induced attacks in AIP mice. AIP mice were induced with a combination of serial phenobarbital injections and diethoxycarbonyl-1,4-dihydrocollidine (DDC) (denoted by arrows [↓]; phenobarbital doses were 90, 100, 110, and 90 mg/kg per day, whereas DDC dose was 20 mg/kg per day) for 4 consecutive days to markedly elevate plasma ALA and PBG concentrations and treated with either a single subcutaneous dose of GalNAc-Alas1 (denoted by a black triangle [▼]; 3 mg/kg), 2 daily infusions of hemin (represented by white triangles [▽]; 4 mg/kg), or saline (controls), as shown. Blood samples were collected (represented by X marks) at the indicated times, and plasma ALA and PBG concentrations were determined. Data shown are means + standard deviations (n = 3-5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/19/10.1182_blood.2022018662/3/m_blood_bld-2022-018662-c-gr5.jpeg?Expires=1770932936&Signature=wJpF1d~nvI21lAv-Nolj2AtpB0fom8aQndG1K7duZHg1kM7rEoG6Gs9CbSDFPvnTObFxfo7N3FH~i8et-taveOOUZaKJHkYakDA1dcAHCv6aigKlspjxg7d9e95bLEweNJx3Olky6dbxGe6Pu7RrQJiUXTTFJnAqkECULZGYWgwzrNrtGDN4zJS1yEuFlz7ilKxi0QrIJ~qLdo1WF91H-NGnkttq6m610w6Iv0lQEmsCEU0i4e61D3SNRRTaysh0NllPjn5Q5ICdUKc2TAHiR2NZi08ZRLDG2gfBZleXwI8FA6yTIphRXZHNp5KBSuLImZglgzcDauPNQVoTptZIzw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![GalNAc-Alas1 effectively prevents phenobarbital-induced attacks in AIP mice. AIP mice were subcutaneously administered GalNAc-Alas1 (denoted by black triangles [▼]; 3 mg/kg per dose) once a week for 4 consecutive weeks and then challenged with serial phenobarbital injections (denoted by arrows [↓]; doses were 110, 120, and 130 mg/kg per day) at weeks (W) 0, 2, 4, and 6 after the last siRNA dose to determine the effectiveness and durability of GalNAc-Alas1 to prevent attacks. Blood samples were collected (depicted by X marks), and plasma ALA and PBG concentrations were determined. Data shown are means + standard deviations. ∗P < .01 and ∗∗P < .005 vs control AIP mice that were phenobarbital-induced after treatment with saline (no GalNAc-Alas1), t-test (n = 3-4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/19/10.1182_blood.2022018662/3/m_blood_bld-2022-018662-c-gr4.jpeg?Expires=1770932937&Signature=z-sVIZydaAjO95dI7cejYHK4xVyv5tlnvOS6ur9NI2KWYIxPsWMwyLIE819zVK8x1LH-SSgTzOFCWkMbzyHtKwsmJV2ub3HOJ-HPmBnLTM02igA9OnKYVIA5kDWWoDgEwv90VOFhl1aSwGgX2XC0je-vd-ShPYfXQ9ec-3Y6gSwjDlxTZ7ikAjw2V069kB-1ZaBVrJ4DH5eZnSQ84kYCJUvFj3wPNQKXFZNzfH5NQIbNk9MHjHx2A6b16t6ZjB3TOIQZNNadPHmk2axC0NPdIrqwO8mJsXXa4we5zawI7nGJwMQI97ggEEAuOe-okrTSyp7GHMzJQ9u4ZEGlSLd9lw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![GalNAc-Alas1 effectively treats ongoing induced attacks in AIP mice. AIP mice were induced with a combination of serial phenobarbital injections and diethoxycarbonyl-1,4-dihydrocollidine (DDC) (denoted by arrows [↓]; phenobarbital doses were 90, 100, 110, and 90 mg/kg per day, whereas DDC dose was 20 mg/kg per day) for 4 consecutive days to markedly elevate plasma ALA and PBG concentrations and treated with either a single subcutaneous dose of GalNAc-Alas1 (denoted by a black triangle [▼]; 3 mg/kg), 2 daily infusions of hemin (represented by white triangles [▽]; 4 mg/kg), or saline (controls), as shown. Blood samples were collected (represented by X marks) at the indicated times, and plasma ALA and PBG concentrations were determined. Data shown are means + standard deviations (n = 3-5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/19/10.1182_blood.2022018662/3/m_blood_bld-2022-018662-c-gr5.jpeg?Expires=1770932937&Signature=a6VnSs1uMvED5OwORSjsRbLHJYog2Eb8X24oaQfj~BsWXYXrguaJoBauvjp99iXAj6cJApk-Yv6WCgAMSw5ZG89rFtXpFCxHuzgWYy51ypw9~qsVNWlQAnSmPEqbwz82SNvp6K7MhOGXEprV1XhM3qCR6b0uuvH15Id3ytYgza6yq1hXzcCxQ23ORfPfIIZIi5lYjBpTjTxB9vlzYzo1Ig4YgnjvXJVXOkjHSGUdl5G5iElIwcJGbEXVw94L8s7ZwQupa9VGoxKGqMJ6B7dqHMOmTDZ-9gtBfHp~VnzV6SUaoSmdEp3RNvZgCsgRnewgI-e0PWKqpmCZrbKzqLPeAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)