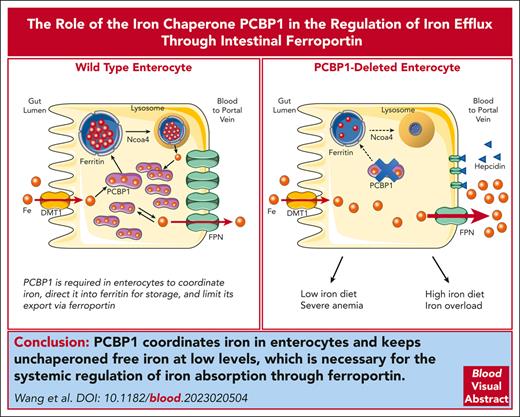
Key Points
The iron chaperone PCBP1 is required in enterocytes to coordinate iron, direct it into ferritin, and limit its export via ferroportin.
Mice lacking PCBP1 in duodenal epithelium fail to properly regulate dietary iron absorption, exposing them to iron overload and deficiency.
Abstract
Iron is an essential nutrient required by all cells but used primarily for red blood cell production. Because humans have no effective mechanism for ridding the body of excess iron, the absorption of dietary iron must be precisely regulated. The critical site of regulation is the transfer of iron from the absorptive enterocyte to the portal circulation via the sole iron efflux transporter, ferroportin. Here, we report that poly(rC)-binding protein 1 (PCBP1), the major cytosolic iron chaperone, is necessary for the regulation of iron flux through ferroportin in the intestine of mice. Mice lacking PCBP1 in the intestinal epithelium exhibit low levels of enterocyte iron, poor retention of dietary iron in enterocyte ferritin, and excess efflux of iron through ferroportin. Excess iron efflux occurred despite lower levels of ferroportin protein in enterocytes and upregulation of the iron regulatory hormone hepcidin. PCBP1 deletion and the resulting unregulated dietary iron absorption led to poor growth, severe anemia on a low-iron diet, and liver oxidative stress with iron loading on a high-iron diet. Ex vivo culture of PCBP1-depleted enteroids demonstrated no defects in hepcidin-mediated ferroportin turnover. However, measurement of kinetically labile iron pools in enteroids competent or blocked for iron efflux indicated that PCBP1 functioned to bind and retain cytosolic iron and limit its availability for ferroportin-mediated efflux. Thus, PCBP1 coordinates enterocyte iron and reduces the concentration of unchaperoned “free” iron to a low level that is necessary for hepcidin-mediated regulation of ferroportin activity.
Introduction
Iron is an essential nutrient because it forms cofactors required in numerous biological processes.1,2 However, iron can be toxic to cells and tissues because of its capacity to produce reactive oxygen species through Fenton/Haber-Weiss chemistry. Because there is no regulated mechanism of iron excretion in humans, intestinal absorption must be strictly regulated to ensure uptake of sufficient iron and to prevent accumulation of excess iron. Most of the iron in the body (>70%) is used to produce hemoglobin for red blood cells. Both insufficient and excess uptake are causes of human diseases such as iron deficiency anemia and hereditary hemochromatosis, respectively.3 Duodenal enterocytes take up dietary iron at their apical membranes through divalent metal ion transporter 1 (DMT1).4-6 Upon entering the enterocyte, intracellular iron may be transferred to ferritin, the iron storage protein, or be exported at basolateral membranes via the efflux transporter, ferroportin (FPN), where it is collected from the lamina propria and delivered into the portal circulation.7-10 Iron stored in enterocyte ferritin may be lost through anoikosis or released intracellularly upon lysosomal degradation and exported to the circulation via FPN. Therefore, the critical step in controlling dietary iron absorption is regulation of iron efflux through FPN.
FPN activity and duodenal iron absorption are regulated at cellular and systemic levels. Intestinal epithelial cells (IECs) rely on the iron regulatory protein system11 and the hypoxia inducible factor 2–α pathway12 to regulate FPN and DMT1 expressions. At the systemic level, intestinal iron absorption is regulated by hepcidin, a peptide hormone primarily synthesized by hepatocytes, which controls the activity of FPN.13,14 Circulating hepcidin binds directly to FPN located on the basolateral membranes of enterocytes, blocking iron efflux and causing FPN internalization and degradation.14 The levels of circulating hepcidin are transcriptionally controlled by signaling molecules that are synthesized and released in response to alterations in body iron requirements. When systemic iron levels rise, expression of hepcidin is elevated, which results in degradation of intestinal FPN and diminished iron absorption.
Upon uptake into the IECs, iron enters kinetically labile cytosolic iron pools. Studies in human cells and murine tissues indicate that labile cytosolic iron is coordinated by a buffering system that includes reduced glutathione and iron chaperones of the poly(rC)-binding protein (PCBP) family.15 PCBPs are multifunctional adaptor proteins that bind cytosine-rich RNA and single-stranded DNA as well as ferrous iron complexes, allowing each ligand to interact with other proteins. PCBP1 was first identified as an iron chaperone because of its capacity to bind and deliver iron to ferritin through direct protein-protein interactions in yeast and mammalian cells.16 PCBP1 is expressed at high levels in mammalian cells.17 It is essential for embryogenesis and fetal development in the mouse18 and plays important roles in iron distribution.19 Cell-based analyses of PCBP1 indicate that it also delivers iron to mononuclear iron centers, such those found in the prolyl and asparagyl hydroxylases that regulate Hif1α,20 and to dinuclear iron centers, such as that of deoxyhypusine hydroxylase.21 PCBP1 can form an iron chaperone complex with BolA2, which allows the transfer of PCBP1-iron to the cytosolic [2Fe-2S] cluster assembly system.22
Studies in mice have revealed distinct, critical functions of PCBP1 in tissues specialized for iron handling. PCBP1-mediated delivery of iron to ferritin is critical in developing erythrocytes, because flux of iron through ferritin is needed for efficient iron delivery to the mitochondria for heme synthesis.23 In hepatocytes, PCBP1 coordination of cellular iron-glutathione complexes is critical for limiting oxidative damage associated with ferroptosis, an iron-dependent mode of cell death caused by iron-mediated lipid oxidation and mitochondrial dysfunction.24,25 Ferroptosis is linked to multiple human diseases, including ischemia-reperfusion injury, neurodegeneration, and cancer.26 Whether iron chaperone activity is required for the absorption of dietary iron in the intestinal epithelium or the maintenance of systemic iron balance in mice has not been explored. Here, we report that mice lacking PCBP1 in the intestinal epithelium fail to store iron in the epithelium and regulate iron efflux through FPN, leading to severe anemia on an iron-limited diet and iron loading and oxidative stress in the liver on an iron-rich diet. Intestinal organoids cultured ex vivo lacking PCBP1 exhibit normal regulation of FPN by hepcidin but enhanced iron efflux and profound depletion of the cytosolic labile iron pool, indicating an uncoupling of normal hepcidin/FPN regulatory systems.
Materials and methods
Refer to supplemental Materials (available on the Blood website) for detailed experimental methods.
Animals and treatments
We crossed C57BL/6J mice carrying PCBP1 flanked by loxP sites (PCBP1fl/fl, or wild type [WT])23 with mice carrying the villin-Cre-ERT2 transgene (gift of Yatrik Shah, University of Michigan)27 to establish the PCBP1fl/fl, Tg villin-CreERT2 line. PCBP1fl/fl females were bred with villin-Cre-ERT2/–, PCBP1fl/fl males. Littermates aged 4 weeks were fed a tamoxifen diet (500 ppm, Envigo) for 1 month to induce intestinal epithelial recombination in villin-Cre-ERT2/–, PCBP1fl/fl mice (PCBP1ΔIEC). Mice were analyzed or fed defined-iron diets (Envigo; “normal iron,” 50 ppm; “low iron,” 2-6 ppm; and “high iron,” 1000 ppm) for additional 3 to 4 weeks. All mouse studies were reviewed and approved by the National Institute of Diabetes and Digestive and Kidney Diseases Animal Care and Use Committee and complied with the National Institutes of Health guidelines for the humane care of animals.
Tissue collection
Assays
Nonheme iron was measured using acid digestion and the chromogen bathophenanthroline disulfonate.28
Immunoblotting was performed using whole enterocyte lysates24 or, for FPN and DMT1, enterocyte membranes29,30 with antibodies listed in supplemental Table 2.
RNA levels were measured using real-time, quantitative polymerase chain reaction, normalizing to GAPDH, GypA, or Rpl19.24 Primers are listed in supplemental Table 1.
For histology, formaldehyde-fixed, paraffin-embedded sections of duodenum were analyzed by hematoxylin and eosin staining and fluorescence immunohistochemistry.24,30,31 Confocal images (3-4) were acquired and mean fluorescence intensity from each image was calculated, background subtracted, and averaged.
Quantitative dietary iron absorption was performed using [57Fe] in a modified established protocol.32,33 Mice were euthanized 1 hour after oral gavage with iron, and 57Fe was measured by inductively coupled plasma mass spectrometry.33 For FPN inhibition, mice received 100 nmol minihepcidin (PR73, gift of Elizabeta Nemeth, UCLA)34,35 or vehicle alone by intraperitoneal injection 3 hours before iron gavage.
Permeability of small intestine was measured using fluorescein isothiocyanate-dextran.36
Enteroids from villin-Cre-ERT2/–, PCBP1fl/fl mice were prepared from small intestine,39 propagated, and differentiated.40 To deplete PCBP1, 5 μM 4-hydroxytamoxifen was added during differentiation. For FPN inhibition, 10 μM minihepcidin was added to the medium, 1 hour before imaging. Labile iron was determined using FerroOrange (Dojindo).41
Statistics
Data were analyzed with Prism 9 (GraphPad Software Inc, San Diego, CA). Results are reported as means ± standard deviation (SD) (N = 8-14). Outliers were identified and excluded using the integrated ROUT method. Differences between 2 groups were analyzed using Welch corrections for unequal variance and unpaired t tests. Differences among groups were determined by 2-way analysis of variance, followed by Bonferroni post hoc analysis. Asterisks ∗∗∗∗, ∗∗∗, ∗∗, and ∗ indicate P < .0001, P < .001, P < .01, P < .05, respectively.
Results
Inducible deletion of PCBP1 in intestinal epithelium
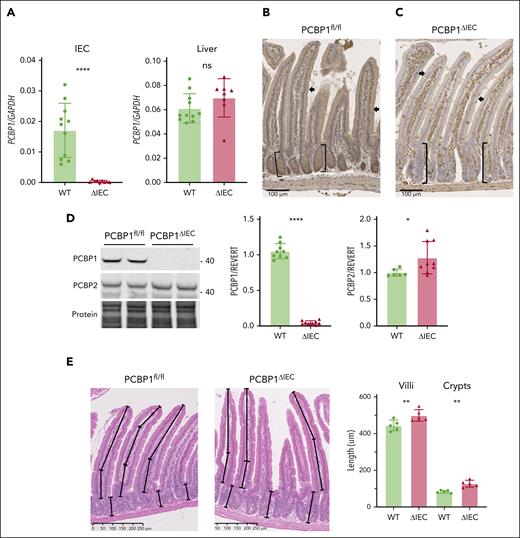
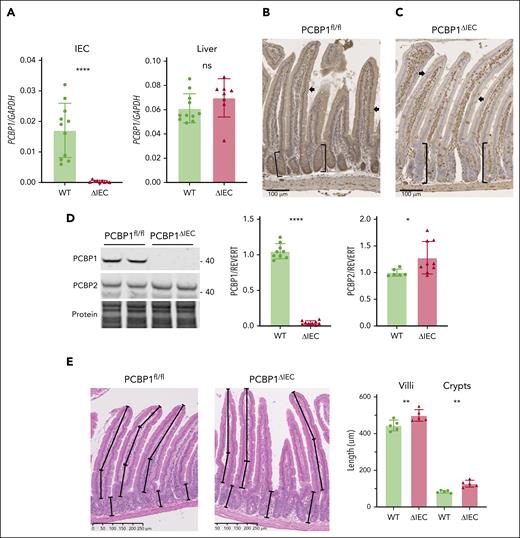
We developed an inducible, enterocyte-specific deletion of PCBP1 using mice carrying the PCBP1 locus flanked by loxP sites (PCBP1fl/fl, WT)23 and mice carrying PCBP1fl/fl and villin-CreERT2 transgene (PCBP1ΔIEC).42 Recombination was induced by feeding offspring a tamoxifen diet. Depletion of PCBP1 messenger RNA (mRNA) in the duodenum of PCBP1ΔIEC mice was confirmed by quantitative polymerase chain reaction of duodenal epithelial scrapings (Figure 1A and supplemental Figure 1). Immunohistochemistry of proximal duodenum demonstrated strong expression of PCBP1 throughout the duodenum of WT mice (Figure 1B). In contrast, PCBP1ΔIEC mice lacked PCBP1 specifically in enterocytes of the villi and crypts but maintained PCBP1 expression in cells of the lamina propria and duodenal wall (Figure 1C). Immunoblot analyses confirmed loss of PCBP1 but not PCBP2 in the IECs (Figure 1D). Although duodenum from PCBP1ΔIEC mice appeared histologically normal, we observed that both villus length and crypt depth of PCBP1ΔIEC mice were 12% and 53% greater than those of WT mice (Figure 1E), with an increased number of proliferating cells (supplemental Figure 1E), suggesting increased rates of proliferation in the stem cell population of the PCBP1ΔIEC crypts.43
Inducible deletion of PCBP1 specifically in the intestinal epithelium of mouse. (A) Relative levels of PCBP1 mRNA in IECs and livers in PCBP1fl/fl (WT) vs PCBP1ΔIEC (ΔIEC) mice. Weanling mice were fed a tamoxifen diet for 1 month before analysis. mRNA levels from IECs of proximal duodenum were determined by real-time quantitative polymerase chain reaction (qPCR). (B) Detection of PCBP1 in all cells throughout the duodenum of WT mice. Fixed, embedded sections of proximal duodenum analyzed by anti-PCBP1 immunohistochemistry. Brown stain indicates PCBP1. Arrows: epithelial cells. Brackets: duodenal crypts. (C) Specific deletion of PCBP1 in duodenal epithelium of ΔIEC mice. PCBP1 detected by IHC as in panel B, above. Note the absence of brown PCBP1 signal in crypts and epithelial layer with PCBP1 expression in lamina propria and duodenal wall. (D) Absence of PCBP1 protein in immunoblot of IECs from PCBP1ΔIEC mice. IECs were collected and analyzed by immunoblotting for PCBP1 and PCBP2. Representative blot shown on the left, quantitation on the right. (E) Increased villus length and crypt depth in PCBP1ΔIEC mice. Intact, full-length villi and crypts were measured in hematoxylin and eosin–stained sections of duodenum. Mean lengths of structures 1 cm distal to pylorus calculated from each animal; 5 age-matched WT and PCBP1ΔIEC mice were analyzed. Refer to female mice data in supplemental Figure 1.
Inducible deletion of PCBP1 specifically in the intestinal epithelium of mouse. (A) Relative levels of PCBP1 mRNA in IECs and livers in PCBP1fl/fl (WT) vs PCBP1ΔIEC (ΔIEC) mice. Weanling mice were fed a tamoxifen diet for 1 month before analysis. mRNA levels from IECs of proximal duodenum were determined by real-time quantitative polymerase chain reaction (qPCR). (B) Detection of PCBP1 in all cells throughout the duodenum of WT mice. Fixed, embedded sections of proximal duodenum analyzed by anti-PCBP1 immunohistochemistry. Brown stain indicates PCBP1. Arrows: epithelial cells. Brackets: duodenal crypts. (C) Specific deletion of PCBP1 in duodenal epithelium of ΔIEC mice. PCBP1 detected by IHC as in panel B, above. Note the absence of brown PCBP1 signal in crypts and epithelial layer with PCBP1 expression in lamina propria and duodenal wall. (D) Absence of PCBP1 protein in immunoblot of IECs from PCBP1ΔIEC mice. IECs were collected and analyzed by immunoblotting for PCBP1 and PCBP2. Representative blot shown on the left, quantitation on the right. (E) Increased villus length and crypt depth in PCBP1ΔIEC mice. Intact, full-length villi and crypts were measured in hematoxylin and eosin–stained sections of duodenum. Mean lengths of structures 1 cm distal to pylorus calculated from each animal; 5 age-matched WT and PCBP1ΔIEC mice were analyzed. Refer to female mice data in supplemental Figure 1.
Impaired growth and iron balance in PCBP1ΔIEC mice
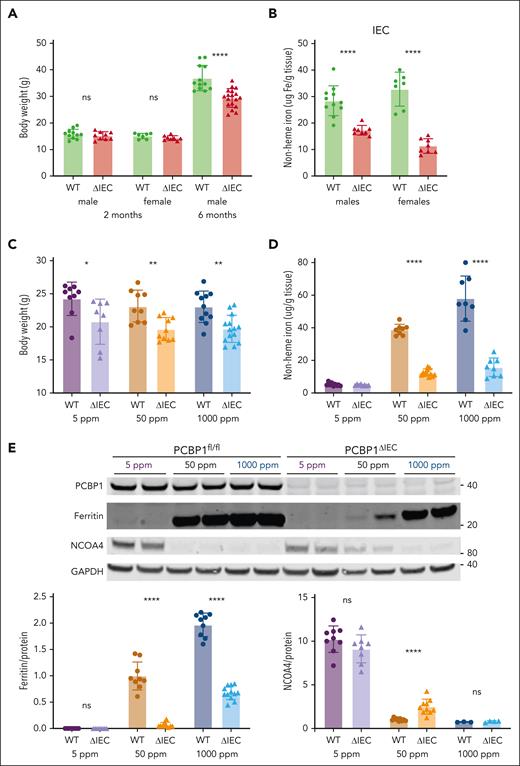
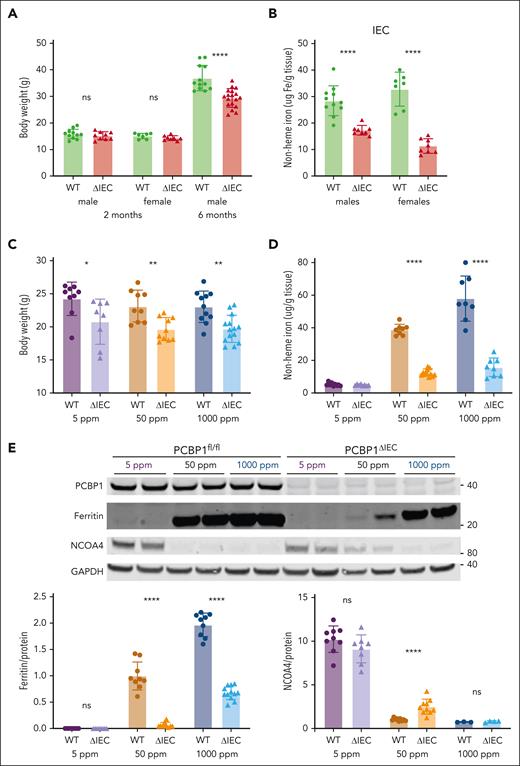
The intestinal epithelium was stressed and possibly impaired in PCBP1ΔIEC mice, therefore, we examined their growth and iron balance. Although younger mice initially grew normally, 6-month-old PCBP1ΔIEC mice had 20% lower body weight than their WT littermates (Figure 2A). Nonheme iron levels in the IECs of young male and female mice were reduced by 39% to 65% (Figure 2B), indicating that loss of PCBP1 disrupted IEC iron balance. To determine the effects of dietary iron on PCBP1ΔIEC mice, they were fed defined-iron diets for an additional 3 to 4 weeks. Dietary iron levels had no effect on body weight in the WT mice; PCBP1ΔIEC mice had 14% to 15% lower body weight vs WT littermates on each defined-iron diet (Figure 2C). We measured iron levels in the IECs and found that the low levels in the PCBP1ΔIEC mice persisted on the defined-iron diets at 68% and 73% below WT in the 50 ppm and 1000 ppm diets, respectively; however the levels were very low and not different from WT on the 5 ppm diet (Figure 2D).
Poor growth and iron depletion in IECs of PCBP1ΔIEC mice. (A) Body weights of WT and PCBP1ΔIEC mice aged 2 and 6 months. Weanling mice fed a tamoxifen diet for 1 month followed by a standard chow diet. (B) Reduced nonheme iron levels in the IECs of PCBP1ΔIEC mice. IECs from 2-month-old mice were collected and analyzed for nonheme iron. (C) Lower body weights of PCBP1ΔIEC mice on defined-iron diets. WT and PCBP1ΔIEC mice were fed 5 ppm, 50 ppm, or 1000 ppm diets for 1 month. Body weight measured at the age 3 months. (D) Reduced nonheme iron levels in the IECs of PCBP1ΔIEC mice on defined-iron diets. IECs from mice in panel C were analyzed for nonheme iron. (E) Lower ferritin and higher NCOA4 levels in IECs of PCBP1ΔIEC vs WT mice. Mice were fed defined-iron diets as in panel C. IECs were collected at 3 months and analyzed by immunoblot for ferritin and NCOA4. A representative blot is shown above with quantitation below. Analyzed mice were male littermates; Female littermates were included as indicated. Refer to data from female mice in supplemental Figure 2.
Poor growth and iron depletion in IECs of PCBP1ΔIEC mice. (A) Body weights of WT and PCBP1ΔIEC mice aged 2 and 6 months. Weanling mice fed a tamoxifen diet for 1 month followed by a standard chow diet. (B) Reduced nonheme iron levels in the IECs of PCBP1ΔIEC mice. IECs from 2-month-old mice were collected and analyzed for nonheme iron. (C) Lower body weights of PCBP1ΔIEC mice on defined-iron diets. WT and PCBP1ΔIEC mice were fed 5 ppm, 50 ppm, or 1000 ppm diets for 1 month. Body weight measured at the age 3 months. (D) Reduced nonheme iron levels in the IECs of PCBP1ΔIEC mice on defined-iron diets. IECs from mice in panel C were analyzed for nonheme iron. (E) Lower ferritin and higher NCOA4 levels in IECs of PCBP1ΔIEC vs WT mice. Mice were fed defined-iron diets as in panel C. IECs were collected at 3 months and analyzed by immunoblot for ferritin and NCOA4. A representative blot is shown above with quantitation below. Analyzed mice were male littermates; Female littermates were included as indicated. Refer to data from female mice in supplemental Figure 2.
We further examined iron homeostasis in the IECs by measuring ferritin and NCOA4 protein levels, which reflect cellular iron storage and regulatory pools. Consistent with the low nonheme iron levels, IECs of PCBP1ΔIEC mice had 94% and 66% lower ferritin than WT when the mice were fed on 50 and 1000 ppm iron diets (Figure 2E;supplemental Figure 2A); whereas ferritin was nearly undetectable in both WT and PCBP1ΔIEC mice that were fed the 5 ppm diet. NCOA4, an autophagosomal cargo receptor for ferritin that varies inversely with regulatory iron pools, exhibited high levels in IECs from mice that were fed the low-iron diet and was low in mice that were fed the high-iron diet, both in the WT and PCBP1ΔIEC mice (Figures 2E; supplemental Figure 2B). However, among mice that were fed the normal-iron diet, we observed 2.5-fold greater NCOA4 levels in PCBP1ΔIEC mice vs WT mice. Changes in the activity of iron regulatory proteins and hypoxia-inducible factors was suggested by the altered iron-regulated transcripts (supplemental Figure 2C-E).29,44 Thus, PCBP1 was critical for maintaining iron homeostasis in the intestinal epithelium.
Increased dietary iron absorption on iron-sufficient diets in PCBP1ΔIEC mice
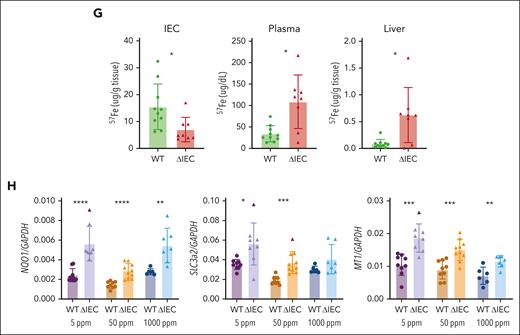
Less iron and less ferritin were stored in the IECs of the PCBP1ΔIEC mice compared with WT mice; whether these differences were due to impaired iron uptake or enhanced efflux was not clear. We measured nonheme iron in plasma and liver and found that plasma iron was 30% and 24% higher in PCBP1ΔIEC mice vs WT mice when fed the normal- or high-iron diets, respectively (Figure 3A). Liver iron levels were elevated in the PCBP1ΔIEC mice that were fed the 1000 ppm diet, with a 47% increase vs WT (Figure 3B; supplemental Figure 3A), and PCBP1ΔIEC mice on a normal diet exhibited a trend toward increased iron in the bone marrow (Figure 3C), suggesting PCBP1ΔIEC mice were exporting more dietary iron and loading iron in the liver.
Severe anemia on iron-deficient diet in PCBP1ΔIEC mice
On the low-iron diet, both WT and PCBP1ΔIEC mice exhibited very low plasma and liver iron levels that did not differ by genotype (Figure 3A-B). This observation, coupled with the very low levels of iron in the IECs of both strains on this diet (Figure 2D-E), suggested both strains had similar levels of iron deficiency and depletion of iron stores. However, PCBP1ΔIEC mice on the low-iron diet exhibited more severe anemia than WT mice, with reduced hemoglobin, hematocrit, mean corpuscular volume, and mean corpuscular hemoglobin (Figure 3D), whereas on the normal diet, they exhibited red cell parameters similar to WT mice (supplemental Figure 3B). Further analysis of bone marrow erythrocyte development indicated impaired maturation of erythrocyte precursors in PCBP1ΔIEC mice (Figure 3E; supplemental Figure 4), consistent with more severe iron-limited erythropoiesis. Transcripts encoding erythroid regulators erythroferrone and erythropoetin were more upregulated in PCBP1ΔIEC mice (Figure 3F). These data suggested that PCBP1 deletion impaired the efficient use of limited dietary iron, possibly by altering storage or absorption kinetics.
To kinetically trace iron absorption in PCBP1ΔIEC mice, we used a stable isotope of iron, 57Fe. We administered 57Fe by oral gavage, collected duodenum, liver, and plasma samples one hour later, and analyzed the samples by ICP-MS. We found that 54% less 57Fe was detected in the duodenal epithelium in PCBP1ΔIEC mice compared with WT; in contrast, 3-fold more 57Fe was detected in the plasma and 7-fold more in liver (Figure 3G). These results suggest that loss of PCBP1 in the IECs leads to enhanced rates of iron efflux from the intestine to the blood. This excess rate of iron efflux led to the sensing of oxidative stress in the liver. The transcription factor Nrf2 is activated by oxidative stress and directs the expression of oxidative stress response genes.24,45 We detected elevated mRNA levels of Nrf2 targets NQO1, SLC3a2, and MT1 in livers of PCBP1ΔIEC mice that were fed defined-iron diets (Figure 3H), which suggested that excess iron efflux in PCBP1ΔIEC mice created oxidative stress in the liver.
Ferroportin mediates the increased iron efflux in PCBP1ΔIEC mice
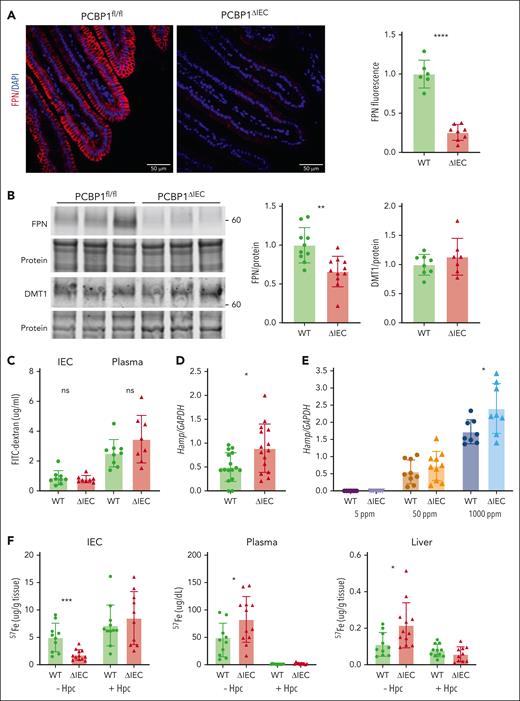
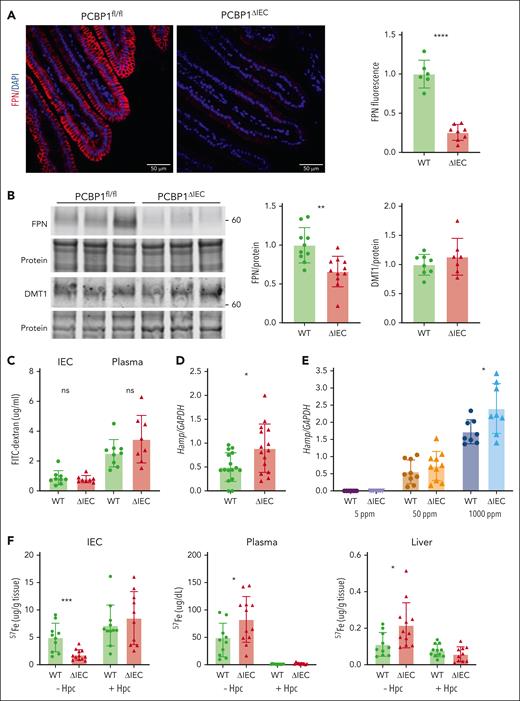
FPN is the sole iron efflux transporter in mammals. FPN efflux activity is normally proportional to the level of FPN expressed on the plasma membrane of iron-exporting cells. The higher rate of iron efflux through the duodenum of PCBP1ΔIEC mice suggested more FPN on the villi of PCBP1ΔIEC mice. Surprisingly, by fluorescent immunohistochemistry, we observed 74% less FPN on the basolateral membrane of enterocytes in the PCBP1ΔIEC mice (Figure 4A; supplemental Figures 5 and 6) and a 34% reduced level of FPN in PCBP1ΔIEC mice by western blotting (Figure 4B) but no significant difference in FPN mRNA (supplemental Figure 2C). Expression of the iron importer DMT was not affected by PCBP1 deletion. We measured the permeability of small intestine using orally administered fluorescein isothiocyanate-dextran and found that dextran levels in the IECs or plasma did not differ between PCBP1ΔIEC and WT mice, indicating epithelial integrity was intact (Figure 4C). Hepcidin is the master regulator of FPN and systemic iron balance; we measured liver hepcidin mRNA (Hamp1) and found that levels were 2-fold elevated in 2 months in PCBP1ΔIEC mice (Figure 4D). In mice that were fed defined-iron diets, liver Hamp1 was appropriately elevated by dietary iron, and higher levels of Hamp1 in PCBP1ΔIEC vs WT liver was observed on the 1000 ppm diet (Figure 4E).
Lower ferroportin levels but higher ferroportin activity in PCBP1ΔIEC mice. (A) Lower levels of ferroportin on duodenal epithelium of PCBP1ΔIEC vs WT mice. Fluorescent immunohistochemistry for FPN in proximal duodenum from 2 months old mice. DAPI (4′,6-diamidino-2-phenylindole) stain indicates nuclei. Representative images on the left show quantitative analysis of fluorescence at right. (B) Lower levels of ferroportin in IECs of PCBP1ΔIEC vs WT mice. IECs from 2 months mice analyzed for FPN and DMT1 by immunoblot. Representative blot on the left, quantitation on the right. (C) Intact tight junctions in intestinal epithelium of PCBP1ΔIEC mice. Mice were administered FITC-dextran (4000 MW) through oral gavage. IECs and plasma were collected after 1 hour and FITC-dextran measured. (D) Higher levels of hepcidin mRNA in livers of PCBP1ΔIEC vs WT mice. Hepcidin mRNA levels in liver were measured by qPCR in 2 months mice. (E) Higher levels of hepcidin mRNA in livers of PCBP1ΔIEC mice on a high-iron diet. Mice were fed defined-iron diets for 1 month and liver hepcidin mRNA levels were analyzed. (F) Ferroportin mediates excess iron absorption through intestinal epithelium of PCBP1ΔIEC mice. Mice were injected intraperitoneally with synthetic hepcidin or vehicle followed by oral gavage with 57Fe solution. IECs, plasma, and liver samples were collected after 1 hour and 57Fe levels measured.
Lower ferroportin levels but higher ferroportin activity in PCBP1ΔIEC mice. (A) Lower levels of ferroportin on duodenal epithelium of PCBP1ΔIEC vs WT mice. Fluorescent immunohistochemistry for FPN in proximal duodenum from 2 months old mice. DAPI (4′,6-diamidino-2-phenylindole) stain indicates nuclei. Representative images on the left show quantitative analysis of fluorescence at right. (B) Lower levels of ferroportin in IECs of PCBP1ΔIEC vs WT mice. IECs from 2 months mice analyzed for FPN and DMT1 by immunoblot. Representative blot on the left, quantitation on the right. (C) Intact tight junctions in intestinal epithelium of PCBP1ΔIEC mice. Mice were administered FITC-dextran (4000 MW) through oral gavage. IECs and plasma were collected after 1 hour and FITC-dextran measured. (D) Higher levels of hepcidin mRNA in livers of PCBP1ΔIEC vs WT mice. Hepcidin mRNA levels in liver were measured by qPCR in 2 months mice. (E) Higher levels of hepcidin mRNA in livers of PCBP1ΔIEC mice on a high-iron diet. Mice were fed defined-iron diets for 1 month and liver hepcidin mRNA levels were analyzed. (F) Ferroportin mediates excess iron absorption through intestinal epithelium of PCBP1ΔIEC mice. Mice were injected intraperitoneally with synthetic hepcidin or vehicle followed by oral gavage with 57Fe solution. IECs, plasma, and liver samples were collected after 1 hour and 57Fe levels measured.
To kinetically analyze iron uptake and efflux through FPN, we used 57Fe in combination with a synthetic mini-hepcidin that completely inhibits FPN activity.34,35 Mice were intraperitoneally injected with hepcidin or the vehicle control before the oral dose of 57Fe, followed by the collection of IECs, plasma, and liver. Similar to Figure 3G, PCBP1ΔIEC mice in the control (without hepcidin) group exhibited 64% less 57Fe in the IECs than WT mice (Figure 4F, left). In contrast, both PCBP1ΔIEC and WT mice pretreated with hepcidin exhibited elevated and equivalent levels of 57Fe in IECs. Thus, blocking efflux with hepcidin revealed that iron uptake into the IEC was not impaired by the deletion of PCBP1. When 57Fe in plasma was measured, again their levels in PCBP1ΔIEC mice were 85% higher than WT mice (Figure 4F, center) and were almost completely suppressed by hepcidin. Levels of 57Fe delivered to liver exhibited a pattern similar to plasma (Figure 4F, right). The apparent discrepancy between the increase in iron efflux activity through FPN and decreased levels of FPN in the duodenum of PCBP1ΔIEC mice could be explained by an increase in the intracellular concentration of “free” ferrous iron, the substrate for FPN.46
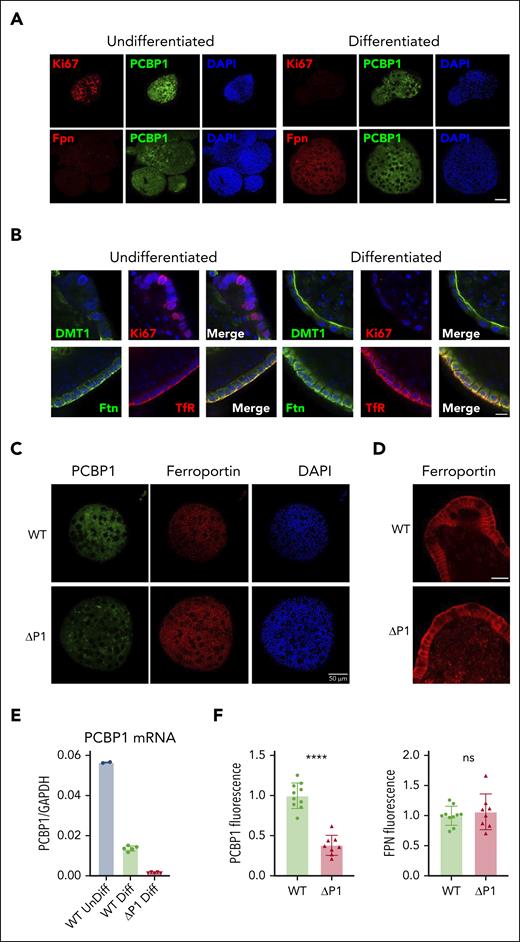
Expression of PCBP1 and ferroportin in ex vivo differentiated enteroids
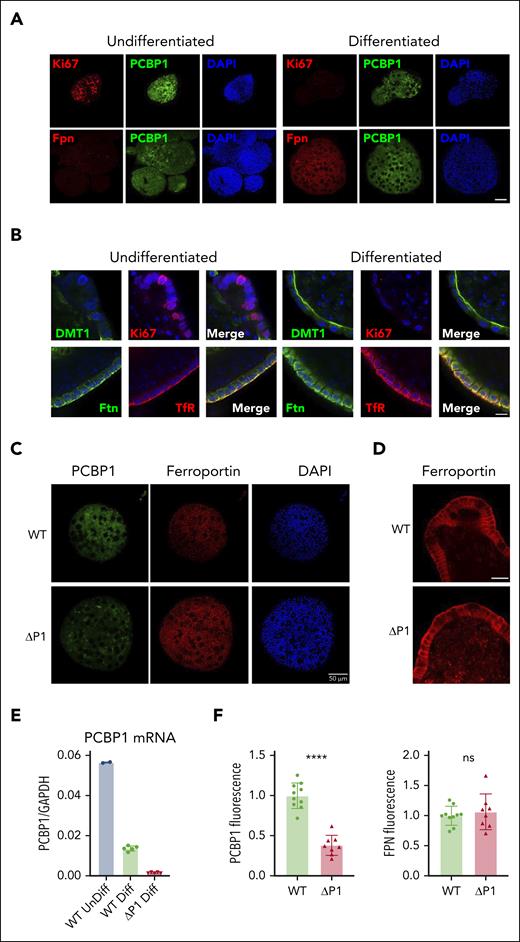
We established 3-dimensional, ex vivo, primary intestinal epithelial cultures (intestinal organoids or enteroids) from PCBP1fl/fl mice to determine whether hepcidin-independent, enterocyte-specific regulatory mechanisms affected FPN activity.47-49 Indirect immunofluorescence on undifferentiated and differentiated WT enteroids confirmed the expression of the proliferation marker Ki67 only in undifferentiated enteroids (Figure 5A,C-D). Both undifferentiated and differentiated enteroids demonstrated strong expression of PCBP1, primarily in the cytosol. FPN was expressed on basolateral membranes of only the differentiated enteroids (Figure 5A,C). DMT1 was primarily expressed in differentiated enteroids and exhibited a strong localization to the apical (interior) membranes. Transferrin receptor 1 and ferritin were expressed in undifferentiated and (more strongly) in differentiated enteroids, exhibiting primarily basolateral membrane/submembrane localization (Figure 5B; supplemental Figure 7).
PCBP1 deletion does not suppress ferroportin expression in differentiated enteroids. (A) Expression of PCBP1 and ferroportin in differentiated WT enteroids. Undifferentiated enteroids on the left, differentiated on the right. Enteroids were fixed and analyzed by indirect immunofluorescence. Proliferation marker Ki67 (red, top panel), FPN (red, bottom panel), PCBP1 (green) and DAPI (blue) are shown. Bar = 50 μm. (B) Expression of key iron homeostatic proteins in differentiated enteroids. Enteroids prepared as in panel A were analyzed for DMT1, Ki67, ferritin (Ftn), and transferrin receptor 1 (TfR) as indicated. DAPI staining in blue. Higher magnification and merged images show distinct subcellular localizations. Bar = 10 μm. (C) Depletion of PCBP1 does not affect FPN expression in the enteroids. PCBP1 depletion was induced with 4-OH tamoxifen in differentiating enteroids from PCBP1fl/fl, TG vil-CreERT2 mice. Indirect immunofluorescence for PCBP1, FPN, and DAPI in WT (no tamoxifen) and ΔPCBP1 (tamoxifen) is shown. Quantitation of fluorescent images is shown below in panel F. (D) No change in basolateral localization of ferroportin in PCBP1-depleted enteroids. Higher magnification images of FPN from central sections in WT and ΔPCBP1 enteroids. Bar = 20 μm. (E) Efficient deletion of PCBP1 in differentiated enteroids. Enteroids treated as in panel C were analyzed by qPCR.
PCBP1 deletion does not suppress ferroportin expression in differentiated enteroids. (A) Expression of PCBP1 and ferroportin in differentiated WT enteroids. Undifferentiated enteroids on the left, differentiated on the right. Enteroids were fixed and analyzed by indirect immunofluorescence. Proliferation marker Ki67 (red, top panel), FPN (red, bottom panel), PCBP1 (green) and DAPI (blue) are shown. Bar = 50 μm. (B) Expression of key iron homeostatic proteins in differentiated enteroids. Enteroids prepared as in panel A were analyzed for DMT1, Ki67, ferritin (Ftn), and transferrin receptor 1 (TfR) as indicated. DAPI staining in blue. Higher magnification and merged images show distinct subcellular localizations. Bar = 10 μm. (C) Depletion of PCBP1 does not affect FPN expression in the enteroids. PCBP1 depletion was induced with 4-OH tamoxifen in differentiating enteroids from PCBP1fl/fl, TG vil-CreERT2 mice. Indirect immunofluorescence for PCBP1, FPN, and DAPI in WT (no tamoxifen) and ΔPCBP1 (tamoxifen) is shown. Quantitation of fluorescent images is shown below in panel F. (D) No change in basolateral localization of ferroportin in PCBP1-depleted enteroids. Higher magnification images of FPN from central sections in WT and ΔPCBP1 enteroids. Bar = 20 μm. (E) Efficient deletion of PCBP1 in differentiated enteroids. Enteroids treated as in panel C were analyzed by qPCR.
Attempts to produce enteroids directly from PCBP1ΔIEC mice were not successful because crypt cells lacking PCBP1 were not viable in ex vivo culture. As an alternative approach, we prepared undifferentiated enteroids from PCBP1fl/fl mice carrying TG villin-CreERT2 and induced recombination with 4-hydroxytamoxifen at the time of ex vivo differentiation. PCBP1-depleted enteroids were studied before the loss of viability. PCBP1 levels substantially decreased after 4-hydroxytamoxifen treatment (Figure 5E-F), however, FPN abundance or localization on basolateral membranes did not change (Figure 5C-D). Thus, the loss of PCBP1 did not trigger FPN repression or degradation in enteroids and suggested that the low levels of FPN in the duodenum of PCBP1ΔIEC mice resulted from systemic regulatory mechanisms.
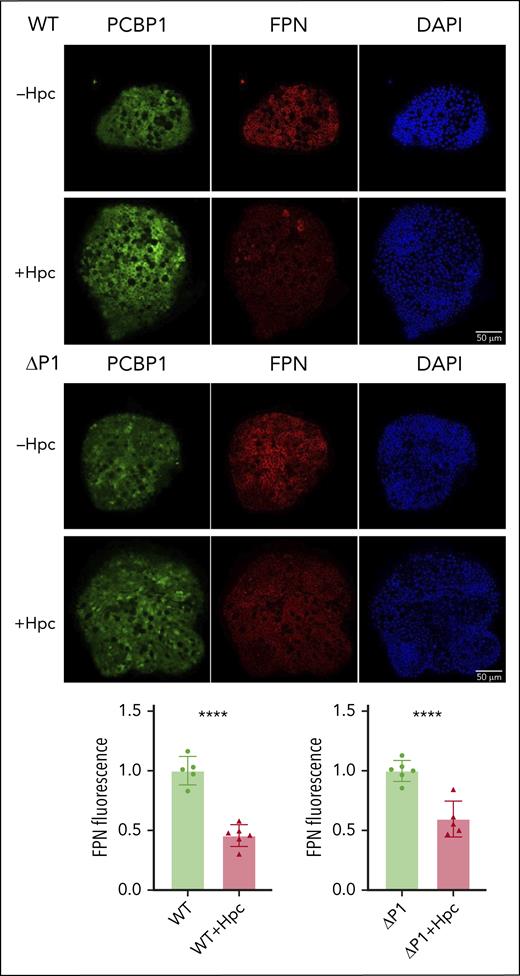
Replication of hepcidin-dependent regulation
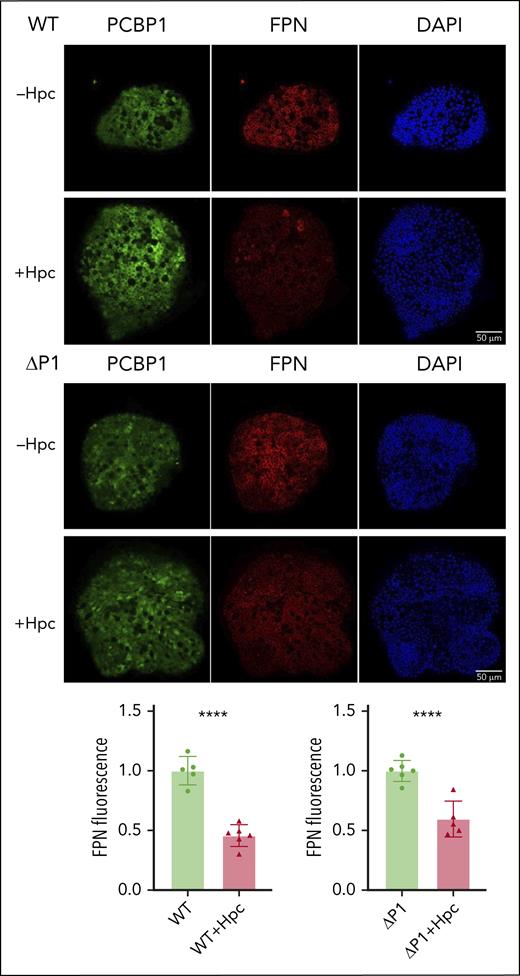
To determine whether hepcidin accounted for the low levels of FPN in the IECs of PCBP1ΔIEC mice, we reproduced this regulatory system ex vivo. Differentiated WT and ΔPCBP1 enteroids were treated with synthetic hepcidin for 18 hours before immunofluorescence analysis. FPN levels were similarly decreased by 54% and 40%, respectively, after hepcidin treatment in WT and ΔPCBP1 enteroids (Figure 6). Thus, hepcidin-triggered degradation of FPN in enteroids occurred similarly in both genotypes.
Hepcidin-mediated degradation of ferroportin in WT and ΔPCBP1 enteroids. WT and ΔPCBP1 differentiated enteroids were treated with hepcidin for 18 hours and fixed and stained for FPN and PCBP1. Quantitation of FPN signals is shown below.
Hepcidin-mediated degradation of ferroportin in WT and ΔPCBP1 enteroids. WT and ΔPCBP1 differentiated enteroids were treated with hepcidin for 18 hours and fixed and stained for FPN and PCBP1. Quantitation of FPN signals is shown below.
Depletion of labile iron in ΔPCBP1 enteroids
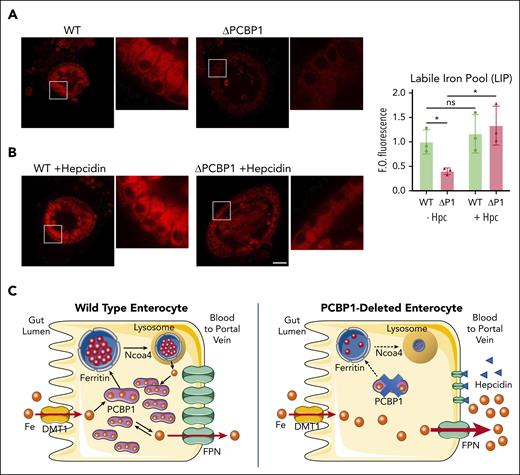
We next considered whether the intracellular iron pools that are substrates for FPN could be affected by PCBP1 deletion. Semiquantitative measurements of intracellular iron pools can be obtained using a cell-permeable fluorescent Fe(II) indicator, FerroOrange (FO).41,50 Although WT enteroids demonstrated a strong FO signal in the cytosol, we detected 60% less signal in ΔPCBP1 enteroids, indicating lower levels of labile iron in enterocytes lacking PCBP1 (Figure 7A). Next, we blocked iron efflux by pretreating enteroids with hepcidin. In WT enteroids, we measured no significant change in FO signal (Figure 7B), indicating that WT enterocytes had no increase in the LIP, suggesting low rates of iron efflux in culture despite strong expression of FPN and abundant cytosolic Fe(II). In contrast, blocking iron efflux in ΔPCBP1 enterocytes caused labile iron pools to rapidly increase to levels similar to WT, indicating that ΔPCBP1 enterocytes initially had low cytosolic Fe(II) because of high rates of iron efflux. WT and ΔPCBP1 enterocytes exhibited dramatically different changes in FO signal with hepcidin treatment, suggesting very different rates of iron efflux even though FPN levels were similar. These observations are consistent with the increased rates of efflux observed in the PCBP1ΔIEC mice in vivo and further suggest the following: (1) the cytosolic labile iron pool is largely coordinated by PCBP1; (2) PCBP1-bound iron is not a substrate for FPN unless and until it dissociates as free iron; (3) unchaperoned, free iron is a substrate for FPN; and (4) PCBP1 is necessary to allow rates of iron efflux through FPN to be controlled by hepcidin on the intestinal epithelium.
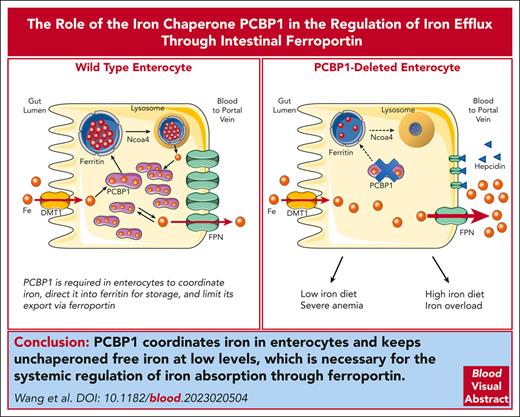
Restoration of labile iron pool in ΔPCBP1 enteroids upon treatment with hepcidin. (A-B) Labile Fe(II) in WT and ΔPCBP1 differentiated enteroids was detected with fluorescent indicator (FerroOrange) without (A) or with (B) hepcidin pretreatment for 1 hour. Central sections of representative enteroids are shown. Images are in pairs with inset at higher magnification on the right. Quantification at right. Enteroids were grown 3 times from individual mice and iron signal from 3 to 7 enteroids in each condition was imaged and measured. Bar = 50 μm. (C) Model of PCBP1-mediated management of cytosolic iron retention and efflux. In WT enterocytes (left), dietary iron imported through DMT1 is rapidly coordinated by PCBP1, which directs excess iron to ferritin for storage and maintains free iron pool at low levels. Lysosomal turnover of ferritin, mediated by NCOA4, occurs when intracellular iron levels are low. Rates of iron efflux through FPN are low and steady. In PCBP1-deleted enterocytes (right), imported iron is not coordinated by PCBP1 and intracellular free iron rapidly rises to high levels. Rates of iron efflux through FPN are high and rapid efflux continues until intracellular free iron falls to low levels. The pool of cytosolic iron buffered by PCBP1 is absent and ferritin storage is low. Iron is initially delivered to portal circulation at a high rate (bolus effect).
Restoration of labile iron pool in ΔPCBP1 enteroids upon treatment with hepcidin. (A-B) Labile Fe(II) in WT and ΔPCBP1 differentiated enteroids was detected with fluorescent indicator (FerroOrange) without (A) or with (B) hepcidin pretreatment for 1 hour. Central sections of representative enteroids are shown. Images are in pairs with inset at higher magnification on the right. Quantification at right. Enteroids were grown 3 times from individual mice and iron signal from 3 to 7 enteroids in each condition was imaged and measured. Bar = 50 μm. (C) Model of PCBP1-mediated management of cytosolic iron retention and efflux. In WT enterocytes (left), dietary iron imported through DMT1 is rapidly coordinated by PCBP1, which directs excess iron to ferritin for storage and maintains free iron pool at low levels. Lysosomal turnover of ferritin, mediated by NCOA4, occurs when intracellular iron levels are low. Rates of iron efflux through FPN are low and steady. In PCBP1-deleted enterocytes (right), imported iron is not coordinated by PCBP1 and intracellular free iron rapidly rises to high levels. Rates of iron efflux through FPN are high and rapid efflux continues until intracellular free iron falls to low levels. The pool of cytosolic iron buffered by PCBP1 is absent and ferritin storage is low. Iron is initially delivered to portal circulation at a high rate (bolus effect).
Discussion
Here, we have demonstrated that PCBP1 plays a critical role in controlling the flux of iron through the duodenal enterocyte. Despite normal levels of iron uptake, IECs lacking PCBP1 contain low amounts of intracellular nonheme iron and low levels of ferritin, indicating that PCBP1 is necessary for the retention of dietary iron within the enterocyte. Furthermore, PCBP1 is essential for hepcidin-mediated regulation of efflux through FPN. Without PCBP1, dietary iron quickly transits the IEC into the systemic circulation. The adverse effects of lack of retention and rapid efflux of IEC iron manifest as poor growth, liver oxidative stress, and liver iron loading when the diet is iron-rich. The adverse effects of PCBP1 deletion also manifest with the iron-poor diet, during which PCBP1ΔIEC mice develop more severe anemia than WT mice. Developing red blood cells rely on the continuous availability of iron to maintain the highly coordinated synthesis of heme and globin chains. Controlled release of iron from the IEC mitigates interruption of the iron supply during periods of fasting, but this capacity is lost when PCBP1 is deleted in the IEC.
Our results are similar to those of a mouse model of intestinal ferritin H deletion, in which the loss of intestinal ferritin was associated with enhanced dietary iron absorption, elevated serum iron, and iron loading of the liver.51 Those studies showed that temporary storage of dietary iron in ferritin allowed the mice to mobilize ferritin iron during periods of fasting and, thereby, maintain a steady flux of iron into the circulation. Both intestinal ferritin- and PCBP1- deletion models demonstrated excess iron loading in liver, indicating that the capacity to store iron in the duodenal epithelium is necessary to appropriately regulate body iron stores. However, intestinal ferritin deletion was not associated with anemia, and the more severe anemia observed in the PCBP1ΔIEC mice on a low-iron diet is likely because of the simultaneous loss of ferritin iron storage and PCBP1 iron buffering. This mouse model also resembles non-HFE hemochromatosis in humans with hepcidin-resistant FPN mutations.52
The high rate of iron efflux in the PCBP1ΔIEC mice was because of a failure in the hepcidin-FPN regulatory axis. Normally, the rate of iron efflux is directly proportional to the amount of FPN on the cell surface. Circulating hepcidin lowers the level of FPN and the rate of iron efflux. Human disorders of iron overload are caused by the inappropriately low levels of hepcidin, which lead to high levels of FPN and excess dietary iron absorption.14 In the PCBP1-deleted enterocytes, however, rates of iron efflux were uncoupled from FPN levels; duodenal FPN was low, but the rate of iron efflux was high. Elevated hepcidin indicated an appropriate regulatory response but was insufficient to suppress iron efflux activity. Our studies using ex vivo cultured enteroids clarified this apparent discrepancy.
In cultured enteroids, PCBP1 depletion revealed the importance of PCBP1 and the LIP in regulating iron efflux. PCBP1 binds Fe(II)-GSH with Kd of 0.4 to 0.8 μM.53 Because the approximate concentrations of PCBP1 (5 μM) and labile iron (0.5-2 μM) in the cytosol are well above this Kd, most of the LIP is likely coordinated by PCBP1, with low free iron levels. Our studies in enteroids, with and without hepcidin treatment, confirmed that the LIP was largely coordinated by PCBP1, free iron levels were low, and both pools were measured by FO. In PCBP1-depleted enteroids, the LIP was much lower than WT, but increased when efflux was blocked, suggesting that Fe(II) levels in PCBP1-depleted enteroids were low because iron was rapidly exported through FPN. Thus, although FPN levels were similar in WT and PCBP1-depleted enteroids, rates of efflux were very different.
What explains this discrepancy? Kinetic studies of purified, recombinant FPN in reconstituted proteoliposomes demonstrate clearly that FPN activity is subject to first-order kinetics in which the rate of efflux increases logarithmically with the concentration of the transported substrate.46 In IECs, free iron levels are low and the rate of efflux is also low, but they can increase in proportion to the number of FPN transporters on the cell surface. In this condition, hepcidin-mediated regulation of FPN levels controls FPN activity. However, if the concentration of free iron is high, such as when PCBP1 is absent, rates of efflux increase logarithmically with the concentration of free iron; the effect of lowering the number of FPN transporters on total efflux is small and hepcidin does not effectively suppress efflux activity. A model depicting this change in regulation is illustrated in Figure 7C. Thus, for hepcidin to effectively regulate FPN activity, the enterocyte requires the iron buffering activity of PCBP1 to maintain free iron at low levels. Previous studies in cells suggest that PCBP2, a paralog of PCBP1, facilitates iron uptake and efflux.54,55 Future studies will clarify the role of PCBP2 in intestinal iron trafficking.
Acknowledgments
The authors thank Nupur Das and Yatrik Shah (University of Michigan), Bryan Mackenzie (University of Cincinnati), Ella Nemeth (University of California, Los Angeles), Tara Arvedson (Amgen), Kostas Pantopoulos (McGill University), Nicholas Zachos, and the Integrated Physiology Core of the Hopkins Conte Digestive Disease Basic and Translational Research Core Center (NIH P30 DK-089502 to N.C.Z.) for generously sharing technical advice and reagents. The authors thank David Bodine (NHGRI), Stacie Anderson and Martha Kirby (NHGRI Flow Cytometry Core Facility), and Deliang Zhang and members of the Rouault laboratory (Eunice Kennedy Shriver National Institute of Child Health and Human Development [NICHD]) for advice and technical assistance.
These studies were supported by the Intramural Research Programs of the National Institutes of Diabetes and Digestive and Kidney Diseases and the NICHD, and the Office of Dietary Supplements Research Scholars Program, Office of the Director, National Institutes of Health.
The authors dedicate this manuscript to the late Manik Ghosh, an excellent scientist and collaborator who made important contributions to these studies.
Authorship
Contribution: Y.W. supported in conceptualization, funding acquisition, and designing methodology, contributed to investigation, and lead the formal analysis, visualization, and writing, reviewing, and editing the original draft; O.P. contributed to the methodology, supervision, and project administration. K.D.H. supported investigation; M.S.-E. supported with investigation and resources; M.C.G. supported investigation and formal analysis; and C.C.P. lead conceptualization, funding acquisition, supervision, and project administration, supported visualization, and contributed to the writing, reviewing, and editing of the original manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.D.H. is Eurofins Lancaster Laboratories, Inc, Lancaster, PA.
The current affiliation for M.S.-E. is Laboratory of Cellular and Developmental Biology, National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD.
Correspondence: Caroline C. Philpott, Genetics and Metabolism Section, Liver Diseases Branch, National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Building 10 Room 9B-16, 10 Center Dr, MSC 1800, Bethesda, MD 20892-1800; e-mail: carolinep@mail.nih.gov.
References
Author notes
Manik C. Ghosh died on 6 August 2021.
Data are available on request from the corresponding author, Caroline C. Philpott (carolinep@intra.niddk.nih.gov).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.