Are hematopoietic cells randomly organized in the human bone marrow? To address this question, in this issue of Blood,Sarachakov et al1 report on their investigation that combined multiparameter tissue staining and artificial intelligence-based image analysis.
Our understanding of the human bone marrow architecture with its distinct niches and their association with hematopoiesis are largely based on mouse and other preclinical models.2 In the vascular niche, hematopoietic stem and progenitor cells (HSPCs) proliferate and differentiate in a highly vascularized microenvironment, surrounded by mesenchymal stem cells.3,4 In contrast, in the hypoxic periosteal niche, osteoblasts have been reported to support HSPC engraftment after transplantation.5
As a step toward a unified human bone marrow niche theory, Sarachakov et al studied 29 individuals with a variety of conditions who had disease-free bone marrow biopsy specimens. Standard bone marrow trephine biopsy samples were stained for precursor myeloid, erythroid, and megakaryocytic cells with fluorescent immunohistochemistry and digitized with whole-slide imaging.
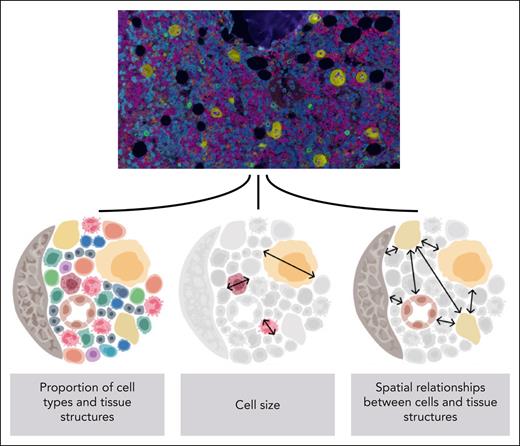
Although trephine biopsies capture only a glimpse of the bone marrow composition, the authors designed and applied a set of advanced computational models to craft this data set into a rich and unique resource (see figure). The end-to-end image analysis pipeline included (1) detection of 1.5 million nucleated cells, (2) their classification by immunophenotype and cellular size into 11 cell types, (3) detection of surrounding histological structures (eg, bone, blood vessels, and fat), and lastly, (4) cell-to-cell and cell-to-structure community analysis to examine their spatial conformity.
Overview of the end-to-end pipeline used in the study. Images (20× objective magnification) of fluorescent immunohistochemistry-stained bone biopsy samples (DAPI, blue; CD34, purple; CD38, orange; CD117, green; CD61, yellow) were analyzed with multiple deep learning-based algorithms to explore the spatial dimension of human hematopoiesis. See Figures 1B and 2C in the article by Sarachakov et al that begins on page 2282.
Overview of the end-to-end pipeline used in the study. Images (20× objective magnification) of fluorescent immunohistochemistry-stained bone biopsy samples (DAPI, blue; CD34, purple; CD38, orange; CD117, green; CD61, yellow) were analyzed with multiple deep learning-based algorithms to explore the spatial dimension of human hematopoiesis. See Figures 1B and 2C in the article by Sarachakov et al that begins on page 2282.
The image analysis tasks were performed mostly with convolutional neural networks.6 These algorithms are based on deep learning and act by encoding images to numeric features, which can be classified to more interpretable histological patterns (eg, cells, blood vessels). Deep learning has been rigorously used in clinical oncology for tasks such as mutation detection from hematoxylin and eosin–stained tissue sections and breast cancer screening from mammography radiographs.7,8 However, the application of these techniques has remained limited in hematology.9 This is likely partly explained by the need for a large number of samples to achieve clinically reliable results but perhaps also by the genomic revolution, which has disrupted the classic morphology-based disease classification of many hematological neoplasms during the past 2 decades.
The authors investigated first how aging influences the bone marrow composition. In line with previous observations, the fat-to-cell ratio was lower in young subjects. Although no association between age and HSPCs were evident, myeloblasts and proerythroblasts were both more frequent and smaller by their cell size in young marrows. These findings raise a captivating follow-up question. Could myeloid precursor cells be more sensitive to aging-related alterations than the stem cells these originate from?
The authors confirmed the occurrence of periosteal and perivascular niches by demonstrating that HSPCs were positioned more proximal to bone and vasculature than would occur by chance. In addition, HSPCs were also commonly located close to megakaryocytes, which were instead located distally to bone and blood vessels. Although HSPC proximity to bone and vasculature did not vary by age, their proximity to megakaryocytes was more frequent in young marrows. Megakaryocytes in younger subjects were also larger in size, but it remains unclear whether HSPCs clustered in the vicinity of large megakaryocytes. Collectively, these results indicate that a distinct megakaryocyte niche could modulate HSPC activity in young marrows. The potential megakaryocytic niche raises intriguing questions for future studies about its role beyond normal hematopoiesis. Could megakaryocytes protect HSPCs from genotoxic insults? Could a decline in megakaryocyte niches be pharmacologically prevented?
Although this study may not have immediate clinical implications, it underscores that at least some hematopoietic cells are not randomly positioned in the human bone marrow and that hematopoiesis is topographically remodeled with age. The diagnostic evaluation and staging of hematological neoplasms are conventionally based on cytomorphology, histopathology, flow cytometry, karyotyping, and genomics. Spatial information such as the proximity of 2 distinct cells or histological structures could reveal important intercellular signaling activity and be translated to clinically relevant prognostic information or treatment biomarkers. Looking ahead, future studies are also necessary to explore remodeling patterns found in different hematological diseases and patient subgroups.
Conflict-of-interest disclosure: O.B. reports research grants from Pfizer and Gilead Sciences, outside the submitted work; reports personal fees from Amgen, Novartis, Sanofi, and Astellas, outside the submitted work; and reports stock ownership (Hematoscope Ltd), outside the submitted work.