In this issue of Blood, Han et al report in vitro studies and an in vivo rat model of polytrauma that demonstrate that leukocyte inflammation contributes to trauma-induced coagulopathy by oxidation and digestion of fibrinogen and that suppression of inflammatory processes blocked the modifications of fibrinogen and trauma-induced coagulopathy.1
Your blood coagulates beautifully.
—Ernest Hemingway,
A Farewell to Arms
Considering that the responses to physical trauma have been fundamental to all vertebrate organisms for millions of years, it is surprising that we know so little about basic mechanisms. In fact, there is evidence that mammalian evolution included the development of a more efficient hemostatic system to deal with trauma.2 Human survival from injury also requires an appropriate inflammatory and immune response.
This research study uses in vitro and in vivo models of polytrauma to gain insight into the effects of inflammation on clotting. Polytrauma is defined as injuries to multiple body parts and organ systems and is associated with hemorrhagic shock as well as increased mortality and morbidity. Major tissue injury and hemorrhagic shock increase inflammation and also contribute to trauma-induced coagulopathy, a major disturbance of coagulation leading to increased bleeding associated with trauma, which greatly elevates early bleeding mortality.
Trauma-induced coagulopathy is a complex process involving many pathways, but loss and consumption of fibrinogen are a cornerstone of the pathology.3 Moreover, it has been previously demonstrated that fibrinogen is both depleted and selectively oxidized in human trauma patients with trauma-induced coagulopathy.4 This article clearly demonstrates that modification of specific regions of fibrinogen through oxidative processes and subsequent degradation is fundamental to trauma-induced coagulopathy.
Fibrinogen is particularly susceptible to oxidation in various environmental conditions. Oxidative changes to fibrinogen have been described, especially those associated with (pro)thrombotic conditions.5 Smokers and patients with myocardial infarction have increased oxidation of fibrinogen, with specific residues in the Bβ chain being nitrosylated.6 Amazingly, even with a very low extent of oxidation of <1 residue per molecule, there are striking effects on fibrin clot structure. Oxidative modifications to fibrinogen as a result of trauma appear to be different from those associated with thrombosis.4
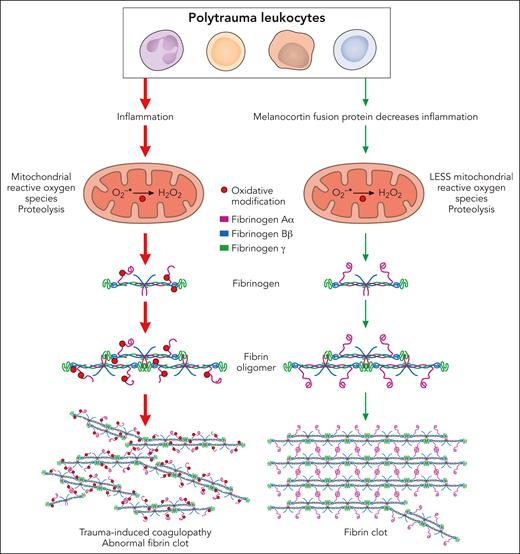
This article defines aspects of immune cell inflammation that play a role in the pathophysiology of acute trauma-induced coagulopathy (see figure). Leukocyte (primarily monocyte) inflammation contributes to trauma-induced coagulopathy by oxidation of the Aα and Bβ chains of fibrinogen and partial degradation of the Aα chains. In vitro, human leukocytes stimulated with interleukin-6 (IL-6) induced cellular inflammation and mitochondrial superoxide, causing fibrinogen oxidation and degradation. Antioxidants suppressing mitochondrial superoxide reduced oxidative stress and inflammation and protected fibrinogen. In rats in vivo, trauma with hemorrhagic shock increased IL-6 and other proinflammatory cytokines and chemokines, selectively oxidized and degraded fibrinogen Aα chains, and caused trauma-induced coagulopathy. The digestion of fibrinogen was not inhibited by tranexamic acid, which inhibits activation of plasminogen to its active form plasmin and has been shown to be effective for treatment of some types of trauma.3
Leukocyte inflammation contributes to the pathophysiology of acute trauma-induced coagulopathy by oxidation and proteolysis of fibrinogen. In both in vitro and in vivo models of trauma, leukocytes generated mitochondrial superoxide, causing oxidation of the Aα and Bβ chains of fibrinogen and partial degradation of the Aα chains, leading to abnormal clot structure. Antioxidants suppressing mitochondrial superoxide reduced inflammation and oxidative stress and protected fibrinogen, preserving fibrin clot structure. Professional illustration by Patrick Lane, ScEYEnce Studios.
Leukocyte inflammation contributes to the pathophysiology of acute trauma-induced coagulopathy by oxidation and proteolysis of fibrinogen. In both in vitro and in vivo models of trauma, leukocytes generated mitochondrial superoxide, causing oxidation of the Aα and Bβ chains of fibrinogen and partial degradation of the Aα chains, leading to abnormal clot structure. Antioxidants suppressing mitochondrial superoxide reduced inflammation and oxidative stress and protected fibrinogen, preserving fibrin clot structure. Professional illustration by Patrick Lane, ScEYEnce Studios.
Of clinical significance, this study suggests that suppression of inflammation by activation of melanocortin pathways may be a novel approach for prevention and treatment of trauma-induced coagulopathy. A newly developed antiinflammatory melanocortin fusion protein (AQB-565), given at the onset of hemorrhage, blocked inflammation, protected fibrinogen from oxidation and degradation, and prevented trauma-induced coagulopathy (see figure).
This report is only the beginning of the story, since this study raises many pressing questions. What are the effects of these modifications of fibrinogen on clot structure and mechanical properties, and how does that lead to increased bleeding? What are the mechanisms and pathways that are involved in these oxidative modifications, and what proteolytic enzymes digest fibrinogen? Will the melanocortin fusion protein be as effective in humans as in rats? Although the degradation of fibrinogen reported here was not inhibited by tranexamic acid, could dual treatment with AQB-565 and tranexamic acid improve patient outcomes? The answers to these and other questions await further research.
Conflict-of-interest disclosure: The author declares no competing financial interests.