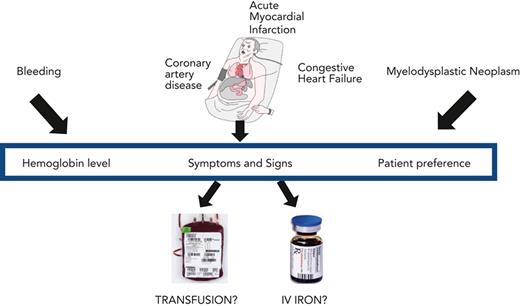
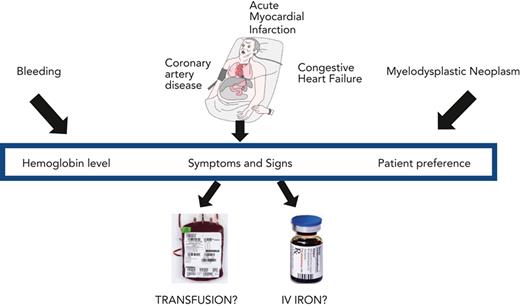
Abstract
Severe anemia is commonly treated with red blood cell transfusion. Clinical trials have demonstrated that a restrictive transfusion strategy of 7 to 8 g/dL is as safe as a liberal transfusion strategy of 9 to 10 g/dL in many clinical settings. Evidence is lacking for subgroups of patients, including those with preexisting coronary artery disease, acute myocardial infarction, congestive heart failure, and myelodysplastic neoplasms. We present 3 clinical vignettes that highlight the clinical challenges in caring for patients with coronary artery disease with gastrointestinal bleeding, congestive heart failure, or myelodysplastic neoplasms. We emphasize that transfusion practice should be guided by patient symptoms and preferences in conjunction with the patient’s hemoglobin concentration. Along with the transfusion decision, evaluation and management of the etiology of the anemia is essential. Iron-restricted erythropoiesis is a common cause of anemia severe enough to be considered for red blood cell transfusion but diagnosis and management of absolute iron deficiency anemia, the anemia of inflammation with functional iron deficiency, or their combination may be problematic. Intravenous iron therapy is generally the treatment of choice for absolute iron deficiency in patients with complex medical disorders, with or without coexisting functional iron deficiency.
Introduction
Red blood cell (RBC) transfusion is one of the most used medical treatments administered in hospitals worldwide. Guidelines for RBC transfusion have been developed across a range of clinical settings1,2 but recommendations are limited for diagnosis and treatment of the causes of anemia that is severe enough to be considered for transfusion. Here, we focus on common causes of anemia sufficiently severe to be considered for transfusion: the anemia of absolute iron deficiency, the anemia of inflammation, and the anemia of myelodysplastic neoplasms. We present 3 selected clinical vignettes that highlight the principles related to transfusion, to the diagnostic evaluation and treatment of associated iron-restricted anemia, and to the initiation of chronic RBC support. We begin with an overview of RBC transfusion, followed by a brief account of iron homeostasis in iron-restricted anemia.
RBC transfusion: efficacy
Significant knowledge has been gained over the past 10 to 20 years through the conduct of large, randomized trials comparing higher vs lower transfusion thresholds in specific patient populations. Higher hemoglobin (Hb) transfusion threshold is referred to as liberal transfusion and lower Hb transfusion thresholds is referred to as restrictive transfusion. In 48 trials (N = 21 433), participants with a variety of diseases and underlying illnesses have been randomly allocated to receive RBC transfusion at a higher Hb concentration of ∼9 to 10 g/dL vs transfusion only when their Hb concentration falls below 7 to 8 g/dL.3 The relative frequency of receiving a transfusion in the restrictive transfusion strategy was 41% lower than the liberal transfusion strategy; risk ratio (RR) 0.59; 95% confidence interval (CI) 0.53 to 0.66.
Analyses across all patients in these randomized trials indicate that restrictive transfusion thresholds ∼7 to 8 g/dL are as safe and effective as the 9 to 10 g/dL threshold, based largely on an analysis of mortality. The 30-day mortality was reported by 31 trials including 16 729 participants. There was no difference in 30-day mortality between restrictive and liberal transfusion strategies; RR 0.99; 95% CI, 0.86 to 1.15.3 No significant differences were found between liberal and restrictive transfusion strategies for multiple morbidity outcomes although the numbers of patients included in these analyses were smaller and the CIs were wider (Table 1).
The evidence supporting transfusion thresholds is less certain in patients with different underlying disorders, including those with cardiovascular disease, gastrointestinal (GI) bleeding, and chronic forms of anemia. The data and clinical issues are discussed in further sections on specific cases. The use of physiological, global, or organ-specific measures that correlate with the need for transfusion await further research.4
Iron-restricted anemia
Effective erythropoiesis requires a sufficient supply of iron for incorporation into Hb for RBC production.5,6 Hepcidin, the iron regulatory hormone, controls delivery of iron to the erythroid marrow by adjusting plasma iron levels.5 Hepcidin interacts with the only known iron exporter, ferroportin, to control the ingress of iron into plasma for transport by transferrin.7,8 Hepcidin increases with inflammation, infection, malignancy, or with elevated body iron stores, retarding iron release. Inflammation can increase circulating hepcidin to concentrations that critically restrict iron recycling and absorption, causing severe anemia.9 Conversely, hepcidin decreases with body iron stores that are inadequate to meet erythroid and tissue iron needs, with hypoxemia, or with heightened demand for erythropoiesis, expanding iron egress through ferroportin and augmenting the supply of plasma iron for erythroid marrow and tissue needs.7 With acute blood loss, renal hypoxia stimulates production of the hormone erythropoietin. Erythropoietin then acts both to expand erythropoiesis and to signal erythroblasts to produce and secrete the hormone erythroferrone. Erythroferrone suppresses hepcidin production by hepatocytes, increasing the iron supply for enhanced erythropoiesis.10,11
Diagnosis of iron-restricted anemia
The reference method for diagnosis of absolute iron deficiency is bone marrow aspiration and biopsy but is rarely needed clinically other than in complex cases. In functional iron deficiency, bone marrow iron stores are present or even increased, except in patients with coexisting absolute iron deficiency. Iron deficiency anemia is usually diagnosed using a complete blood count with peripheral blood smear and iron studies12 (Table 2). The transferrin saturation, providing a measure of the current iron supply for erythroid and tissue needs, can be decreased by either absolute or functional iron deficiency. The serum ferritin concentration is a good measure of iron stores in healthy individuals, but ferritin is an acute phase protein that is increased by coexisting inflammation, limiting its usefulness in patients with complex medical disorders. The serum soluble transferrin receptor concentration is much less affected by inflammation. The test may be especially helpful in patients with complex medical disorders at risk for both absolute and functional iron deficiency anemia, but it is not available as an inhouse test in many clinical laboratories.
Treatment of iron-restricted anemia
Before beginning treatment of iron-restricted anemia, a crucial task is to identify and, if possible, remedy or treat the underlying etiology. For otherwise healthy patients, oral iron therapy is the treatment of choice for absolute iron deficiency anemia because of its safety, effectiveness, and low cost. In patients with anemia so severe as to be considered for transfusion, absolute iron deficiency is seldom uncomplicated and intravenous iron is generally the treatment of choice for absolute iron deficiency alone or with coexistent functional iron deficiency9 (refer to further sections).
Case 1: GI bleeding in a patient with history of coronary artery disease
The patient was a 68-year-old male with a medical history of hypertension and coronary artery disease. He underwent percutaneous coronary intervention 8 months previously. He had been treated with aspirin, clopidogrel, atorvastatin, and chlorthalidone. He presented to the emergency room with 2 episodes of hematemesis and complained of being lightheaded on standing. He gave a history of postprandial epigastric discomfort and denied chest pain or shortness of breath. Physical examination was significant for a diaphoretic male, blood pressure, 90/60 mm Hg; pulse, 118 beats per minute; respiratory rate, 17 breaths per minute. Examination was normal with the exceptions that conjunctiva was pale and minimal epigastric tenderness was present. Stool was black and hemoccult positive. Hb was 9.1; MCV, 70 fL; platelet count, 380 × 103/μL, and white blood cell count was 8.2 × 103/μL. Microcytosis and hypochromia were seen in the peripheral blood smear. First troponin was normal. Electrocardiogram was unchanged from 8 months ago. The patient received 2 L saline IV and within 1 hour his blood pressure was 130/70 mm Hg and his pulse was 80 beats per minute, and he reported that he had felt much better. Four hours later the Hb was 8.1 g/dL, and 8 hours later it was 7.4 g/dL. Repeat troponin level was normal. He had complained of being weak but denied chest pain.
Case 1: comment
RBC transfusion
The obvious source of the patient’s acute anemia was upper GI bleeding but management was challenging. The patient presented with orthostatic symptoms, hypotension, and tachycardia. He was promptly resuscitated with IV fluids and typed and crossed for several units of blood. The question arises: what clinical parameters and Hb level should trigger RBC transfusion(s) in a patient with GI bleeding and underlying coronary artery disease?
Patients with GI bleeding should be managed with a restrictive transfusion strategy because trials show that mortality is no higher and may be slightly lower than with a liberal transfusion strategy. The only trial to demonstrate harm from liberal transfusion was conducted in participants with upper GI bleeding who were randomly allocated to a restrictive transfusion strategy with a 7 g/dL threshold or to a liberal transfusion strategy of 9 g/dL.13 Mortality was lower in the restrictive transfusion strategy; hazard ratio 0.55; 95% CI, 0.33 to 0.92. Recurrent bleeding and portal pressures were also lower in the restrictive transfusion group. In meta-analysis including 3 trials, restrictive transfusion was associated with lower all-cause mortality (RR = 0.65; 95% CI, 0.43-0.97)3 and rebleeding.14 Further study is needed to determine whether mortality differs between bleeding from peptic ulcer disease or portal hypertension due to cirrhosis.
The problem with applying the aforementioned trial evidence to the patient in case 1 is twofold. First, the GI bleeding trial that found lower mortality in the restrictive transfusion arm excluded patients with cardiac disease and the patient in case 1 had established coronary artery disease. Second, in a patient that is bleeding, estimating the nadir Hb level may be very difficult especially in a patient with hemodynamic instability. Development of more severe (<6 g/dL) anemia15 could be dangerous for a patient with coronary artery disease.
Optimal management of acute anemia in patients with coronary artery disease is unclear. Anemia may be deleterious in patients with cardiovascular disease because oxygen delivery to the myocardium is flow dependent because the heart extracts a high percentage of oxygen. Therefore, myocardial ischemia may be precipitated or worsened by low Hb concentrations, especially in patients with coronary stenosis or active plaques. Studies performed with canines16-18 and patients who decline blood transfusion for religious reasons suggest a decreased ability to tolerate anemia in the presence of coronary artery disease.15
Management of acute anemia varies depending on the underlying cardiovascular disease and prior treatment. We categorize patients into 3 groups: (1) preexisting coronary artery disease, (2) acute myocardial infarction, and (3) patients undergoing coronary artery bypass. In the clinical setting of coronary artery bypass surgery, 4 trials in 7441 participants have demonstrated the safety of a restrictive transfusion strategy on 30-day mortality (RR 0.99; 95% CI, 0.74-1.33).3,19-23 The largest trial in >5000 patients used a restrictive transfusion threshold of 7.5 g/dL.22,23 However, because the obstructed coronary arteries have been bypassed, these data do not pertain to the patient in case 1.
Patients with preexisting cardiovascular disease have been included in many trials. One trial, which enrolled 2016 patients with cardiovascular disease or cardiovascular risk factors, found no difference in clinical or functional outcomes.24 Other small trials had similar results.25-28
However, many patients with cardiovascular disease are not identified in reports from trials recruiting in a general medical or surgical setting. Thus, significant uncertainty remains as to the optimal transfusion strategy for patients, like in case 1, with preexisting cardiovascular disease.29,30
Uncertainty also remains as to the optimal transfusion strategy in acute myocardial infarction. Three trials, including a total of 822 patients with acute myocardial infarction, have compared restrictive and liberal transfusion strategies.31-33 When combined in meta-analysis, the relative risk for 30-day mortality was 1.61 (95% CI, 0.38-6.88), favoring lower mortality rate in liberal transfusion.3 A 3500-patient trial called Myocardial Ischemia and Transfusion is currently enrolling patients with acute myocardial infarction and a Hb concentration of <10 g/dL and comparing liberal transfusion strategy of 10 g/dL with a restrictive transfusion strategy that permits, but does not require, transfusion when the Hb concentration is <8 g/dL. This trial should report results in 1 to 2 years.34
Clinical judgment is required given the uncertainty surrounding the optimal transfusion approach with GI bleeding and coronary disease. A careful history should focus on symptoms of anemia, angina, and further bleeding. The priority should be to reduce the probability of myocardial ischemia rather than transfusion avoidance. In case 1, given the patient’s last Hb of 7.4 g/dL, we would not want to risk a lower Hb level, would transfuse 1 unit of blood, and then reassess even in the absence of symptoms.
After transfusion of 1 unit of blood, the patient was hemodynamically stable with Hb of 8.4 g/dL. IV proton pump inhibitor therapy was initiated. Upper endoscopy found a gastric ulcer. Laboratory studies reported a transferrin saturation of 15%, total iron-binding capacity (TIBC) of 470 mg/dL, ferritin of 12 μg/L, and serum soluble transferrin receptor of 7.3 mg/L (reference range, 2.2-5.0 mg/L). Serum creatinine, C-reactive protein (CRP), vitamin B12, and folate levels were normal.
Underlying anemia
With GI bleeding as in case 1, diagnosis and therapy for the anemia underlying the potential need for RBC transfusion are vital but may not be undertaken in practice. A retrospective review of patients with GI bleeding at a tertiary care center found that >90% of patients were anemic but only a minority had any evaluation or treatment for iron deficiency.35
For the patient in case 1, the acute upper GI bleed was superimposed on chronic blood loss that had led to absolute iron deficiency, as evidenced by the peripheral blood smear and laboratory results. Both oral and IV iron therapy could be considered. A randomized trial in patients admitted to hospital with nonvariceal acute upper GI bleeding, as in the patient in case 1, found no significant difference in increasing Hb concentrations between oral and IV iron (P = .46), but oral iron was less effective in replenishing iron stores (P < .05).36 In deciding between oral and IV iron therapy, a recent systematic review and meta-analysis of 154 randomized clinical trials that included 32 762 participants found that IV iron was associated with an increased risk of infection when compared with oral iron or no iron (RR, 1.16; 95% CI, 1.03-1.29).37 In addition, IV iron was associated with a reduction in the risk of requiring a RBC transfusion (RR, 0.93; 95% CI, 0.76-0.89) when compared with oral iron or no iron. Although mindful of the evidence for an increase in the risk of infection, we would opt for high-dose intravenous over oral iron therapy in this patient, given the reduced risk of requiring a RBC transfusion, better replenishment of iron stores, the risk of GI symptoms resulting in poor adherence to oral iron, and interference with oral iron absorption from continued proton pump inhibitor therapy. Four IV iron preparations approved in the United States and in Europe may be given by single, high-dose administration (Table 3) and are similarly effective in treating iron deficiency. The risks of anaphylactic reactions are very low38 and serious adverse events are uncommon.39
Case 2: congestive heart failure (CHF)
The patient was a 73-year-old woman with a long history of poorly controlled hypertension. In the past 2 years she had developed increasing dyspnea, orthopnea, and peripheral edema. Her echocardiogram showed global left ventricular dysfunction; the ejection fraction was 30%. Stress test was negative for ischemia. On peripheral blood smear, RBCs were normocytic and normochromic. Hb was 8.4 g/dL; MCV, 90 fL; serum iron, 80 μg/dL; TIBC, 320 mg/dL, transferrin saturation, 25%; ferritin, 86 μg/dL; serum soluble transferrin receptor, 4.2 (reference range 2.2-5.0 mg/L); serum creatinine, 2.0 mg/dL; and CRP, 12 mg/L. Vitamin B12, folate, and thyrotropin were normal. Stool hemoccult was negative. She was treated with furosemide and sacubitril/valsartan, and her edema resolved. With diuresis, the patient’s Hb concentration increased to 9.2 g/dL. She had minimal dyspnea on exertion.
Case 2: comment
RBC transfusion
Treatment of patients that are anemic with CHF is challenging because clinicians must balance potential benefit of transfusion resulting from better oxygen delivery from higher Hb levels with risk of worsening congestive symptoms by administering blood to a volume-overloaded patient. No published trials have compared transfusion thresholds in patients with CHF. RBC transfusion is not indicated in the patient in case 2 because the Hb level was not sufficiently low. If the Hb concentration were to fall to between 7.0 and 8.0 g/dL or if symptoms developed, transfusion should be considered. The overall risk of transfusion-associated cardiac overload is ∼1% to 3%,40,41 but probably substantially higher with CHF. Transfusing a patient with poor left ventricular function requires blood to be administered slowly and may include prescribing diuretics before and after the transfusion.
Underlying anemia
Anemia is common in CHF, present in one-third to one-half of patients, and is independently associated with both a decreased quality of life and increased mortality.42,43 Treatment of anemia with erythropoietin-stimulating agents in patients with heart failure was abandoned after finding no improvement in cardiovascular outcomes but increases in thromboembolic events and ischemic stroke.44 Treatment of iron deficiency, among the most common disorders contributing to anemia in CHF, has been intensively investigated, both for effects on Hb concentration and on cardiac outcomes. Meta-analyses of randomized clinical trials in the 50% to 80% of adults with chronic CHF who are iron deficient have found that IV iron significantly reduces recurrent hospitalization but does not decrease all-cause mortality.45,46 A trial of oral iron therapy found no significant effects.47 The American College of Cardiology (ACC), the American Heart Association (AHA), and the European Society of Cardiology (ESC) guidelines recommend consideration of IV iron therapy for adult patients who are iron deficient with chronic heart failure, with or without anemia.48,49 The criteria for the definition of iron deficiency (serum ferritin < 100 μg/L alone, or 100-300 μg/L with a transferrin saturation of <20%) recommended by these guidelines have been used in most epidemiological studies and clinical trials.
Nonetheless, the optimal criteria for iron deficiency remain uncertain.50 In adults, using the reference standard of bone marrow examination, a transferrin saturation of <20% has a sensitivity of 94% and specificity of 84% for absolute iron deficiency,51 both significantly higher than using criteria recommended in the ACC/AHA/ESC guidelines. The guideline criteria were originally derived from trials of IV iron in adults with end-stage renal failure in which the etiology of iron deficiency is different from that in heart failure.52
Other studies have supported the use of a transferrin saturation of <20% as the criterion for absolute iron deficiency that is most closely linked to outcomes in adult heart failure.52-57
Altogether, these data suggest that a serum ferritin concentration of <100 μg/dL with a transferrin saturation of >20% identifies patients with heart failure who have a functional iron deficiency with an inflammatory increase in serum ferritin and without absolute iron deficiency. In case 2, ACC/AHA/ESC guidelines would suggest consideration of administration of IV iron, given that the serum ferritin concentration was <100 μg/L. We would interpret the transferrin saturation of >20% as evidence against absolute iron deficiency and would not treat with IV iron. We also had available a normal serum soluble transferrin receptor concentration that provided more evidence for the absence of absolute iron deficiency in this complex case.58
Clinically, if the patient in case 2 were stable and asymptomatic, we would discharge and follow-up within 1 month. In patients admitted with acute heart failure, functional iron deficiency may improve or resolve within weeks.59,60 We would then reassess the change in Hb concentration and determine the need for further evaluation.
Case 3: myelodysplastic neoplasm
The patient was a 76-year-old male with a diagnosis of lower-risk myelodysplastic syndrome (MDS) with ring sideroblasts treated with luspatercept who had been admitted with complaints of fatigue and difficulty ambulating at home. Up until 6 months before hospital admission, the patient had been exercising daily on a treadmill. Physical examination was normal except for pale conjunctiva. Laboratory evaluation found Hb, 7.3 g/dL; MCV, 94 fL; white blood cell count, 6.5 × 103/μL with normal differential; platelet count, 325 × 103/μL, normal peripheral smear, and a reticulocyte count of 1.0%. Creatinine was 1.8 mg/dL and estimated glomerular filtration rate was 35. Serum iron, 75 μg/dL; TIBC, 225 mg/dL; transferrin saturation, 33%; ferritin, 120 μg/dL; serum soluble transferrin receptor, 3.8 mg/L (reference range 2.2-5.0 mg/L); erythropoietin, 825 mU/mL; and serum CRP, 12 mg/L. Vitamin B12, folate, and thyrotropin were normal. Serum protein electrophoresis, immunofixation, quantitative immunoglobulins, and serum-free light chain assay were normal. Bone marrow aspirate revealed dysplastic erythropoiesis with 6% ringed sideroblasts and atypical megakaryocytes. The myeloblast percentage was 4%. There were no immunophenotypic abnormalities. The bone marrow biopsy indicated a hypercellular bone marrow (80%) with no fibrosis. The cytogenetics and MDS fluorescence in situ hybridization profile indicated no abnormalities, and next-generation sequencing with a myeloid mutation profile revealed a mutation in SF3B1. Stool hemoccult was negative. The Hb level 2 and 4 years ago was 11.7 g/dL and 13.3 g/dL, respectively. No anemia workup was conducted 2 years ago.
Case 3: comment
The patient’s history and physical examination suggest slow onset of anemia with declining exercise tolerance and an increase in fatigue. Erythropoietin-stimulating agent was not prescribed because erythropoietin level was >500 mU/mL.61 The patient seemed to have had become refractory to luspatercept therapy. We restrict this commentary to considerations related to initiation of chronic RBC transfusion.
The goals of transfusion are to improve symptoms of anemia and avoid the complications of severe anemia.62 The level of anemia and need for transfusion can be a marker for disease severity. The optimal threshold is unknown in patients with MDS and is likely to need to be highly individualized based on the diverse patient characteristics, disease states, and treatment options in MDS. Only 2 small pilot trials (n = 19 and 38) in patients with MDS have been published.63,64 The study with 38 patients indicated possible improvement in some quality of life measures with a liberal transfusion policy (10.5 g/dL compared with 8.5 g/dL). Surveys of hematologists indicate widely divergent practices.65,66
In the absence of more conclusive trial data, we would administer RBC transfusion to the patient in case 3. Before his severe anemia, he was highly functioning and exercised regularly. We would try to establish goals related to his desired activity level and ask about the importance of participation in other physical activities. We would administer 2 units of blood, measure his post-transfusion Hb level, and carefully assess his level of fatigue and activity level. We would ask if he felt well enough to exercise and to take walks near his home. His post-transfusion Hb level was 9.1 g/dL and he had reported partial improvement in his symptoms, but he did not feel well enough to resume his physical activities. Therefore, we gave him 1 more unit of RBC, measured his post-transfusion Hb (10.2 g/dL), and queried him on how he felt and what activities he felt well enough to perform. The goal would be to learn whether there was a reasonable target Hb that he appeared to prefer. Subsequent transfusion practice would then be informed by the frequency and tolerance of transfusion needed to sustain clinical improvement in conjunction with the trajectory of his disease and the impact of any new MDS-specific therapies engaged.
This approach is consistent with findings from a survey of 447 patients with diagnosis of MDS, who had received a transfusion in the past 8 weeks.67,68 The respondents had a diagnosis of MDS for a median of 3 years (interquartile range, 1-6 years) and a mean age of 73 (range, 67-79 years). The most frequent symptoms before transfusion were fatigue, weakness, and dyspnea. They felt better most frequently 1 to 2 days after transfusion, although 7% did not improve. Twenty percent felt worse for 1 to 2 days after transfusion. Most patients who noted improvement indicated they felt better for at least 5 days. The most frequent request of patients was for point of care home testing of Hb level. Importantly, 26% preferred use of higher transfusion thresholds to what was being employed by their physicians, 62% preferred a threshold of 8.5 g/dL and 20% a threshold of 10 g/dL.
Therefore, we would rediscuss with the patient the goals of treatment and the risks associated with multiple transfusions including transfusion-associated cardiac overload, alloimmunization, and iron overload.69-71 We would communicate that the beneficial effect of transfusion is transient, lasting as long as the transfused RBCs. It may not be possible or advisable to administer enough blood to a patient to optimize how they feel. So many transfusions may be required that the risk of adverse effects of transfusion would be significant. Exercising regularly is a worthy goal but, clearly, may not be worth the risk. In contrast, transfusions to maintain a Hb level high enough to eliminate angina would be an important goal. Patients may not feel better after transfusion because the underlying illness is responsible for the symptoms rather than the level of anemia. This would lead to the use of a lower Hb target and fewer transfusions. Each transfusion requires time from home and family and can negatively impact quality of life.
Many factors besides Hb level must be considered in transfusion decisions, including but not limited to life expectancy, ease of traveling to hospital for transfusion, side effects of transfusion (including iron overload and alloimmunization), difficulty in finding a matched unit of blood, duration of benefit, and patient preference. These factors must all be discussed with the patient and a shared decision is essential in making a final decision.
Summary
Clinical trials have clearly demonstrated that a restrictive transfusion strategy of 7 to 8 g/dL is safe. Our cases have highlighted common clinical settings in which there is a dearth of high-quality evidence to guide transfusion practice. Furthermore, although trials have focused on Hb thresholds to guide transfusion, we have emphasized that incorporation of careful assessment of a patient’s signs, symptoms, comorbidity, and disease trajectory is essential in transfusion decisions. Clinical trials provide an average effect in a population of patients, but each patient has their own unique physiology, symptoms, projected clinical course, and preferences. It is therefore essential to individualize transfusion therapy on the foundation of the safety of a restrictive policy.
Acknowledgments
The authors are grateful to Roger Strair for reviewing the manuscript.
This study was supported by the National Institutes of Health, National Heart Lung and Blood Institute (U01 HL 133817) (J.L.C.), the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK11544 and R01 DK116126), and the US Food and Drug Administration (R01 FD006372) (G.M.B.).
Authorship
Contribution: J.L.C. and G.M.B. wrote the manuscript; J.L.C. focused on the RBC transfusion sections and G.M.B. focused on the iron sections.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey L. Carson, Department of Medicine, Rutgers Robert Wood Johnson Medical School, 125 Paterson St, New Brunswick, NJ 08901; e-mail: jeffrey.carson@rutgers.edu.