Background and objectives
Hemophilia A is an inherited bleeding disorder characterized by a deficiency in blood clotting factor VIII (FVIII). Its severity varies based on the degree of FVIII deficiency, with the most severe cases experiencing frequent spontaneous hemorrhages (FVIII levels < 1 IU/dL). Treatment options include on-demand administration following a hemorrhage or prophylaxis to prevent bleeding. The most frequent complication is the production of inhibitory antibodies against the coagulation factors, leading to more aggressive and expensive treatments.
The aim of this study was to describe Hemophilia A patients according to severity in France in 2021, including sociodemographic characteristics and comorbidities, current treatments, healthcare resource use and related costs.
Methods
This study used the French health-insurance claims database (SNDS) containing pseudonymized individual data for over 66 million people. Hemophilia A patients are fully covered in France and might be identified by the ICD-10 code D66. Patients alive on January 1st, 2021, were selected after exclusion of those presenting with Willebrand disease and other rare bleeding disorders. Comorbidities were identified through validated algorithms. An age and gender matched control group without Hemophilia A was randomly selected in the general population: for each HA patient a subject of the same age, gender and region of residence was randomly selected among the overall population of French citizens (excluding HA patients). Sub-group analyses were performed according to the treatment pattern (on demand / in prophylaxis) and the presence of inhibitors (defined with the treatment: emicizumab before October 2019, high dose of factor VIII or bypassing agents). Direct costs were estimated in a societal perspective.
Results
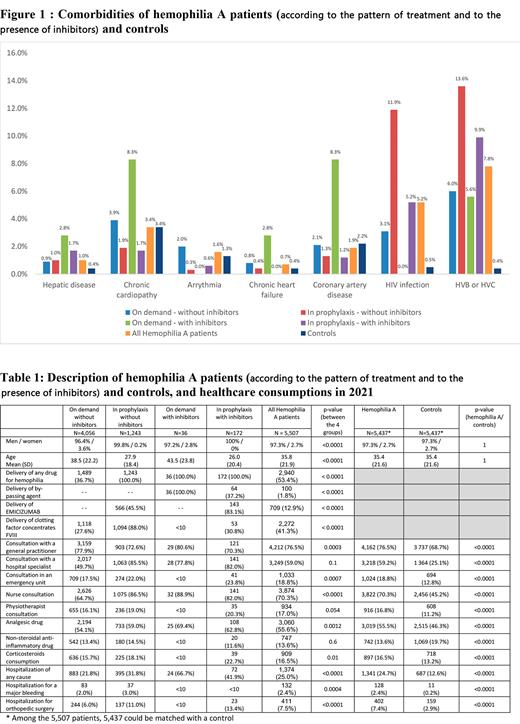
5,507 patients with Hemophilia A were identified. Mean age was 36 years and 97.3% were males.
Compared to controls, hemophilia patients had significantly (p<0.05) more frequently an HIV infection, hepatitis B or C, and a hepatic disease (Figure 1). There were no significant differences for chronic cardiopathy, coronary artery disease, chronic heart failure and arrhythmia.
Respectively 4,056 (73.7%), 36 (0.7%), 1,243 (22.6%) and 172 (3.1%) patients were treated on demand without and with inhibitors and in prophylaxis without and with inhibitors.
Compared to controls, patients with hemophilia A had more frequent consultations with general practitioners and hospital specialists, visits to emergency unit, with nurses and physiotherapists (Table 1). They also were more frequently treated with analgesics and corticosteroids but less frequently with non-steroid anti-inflammatory drugs. The proportion of hemophilia A patients hospitalized was higher than controls, overall and also for bleeding episodes and for orthopedic surgery.
The mean annual direct medical costs (drugs, consultations, hospitalizations, ...) varied strongly according to treatment modalities and presence of inhibitors:
€11,471 for patients treated on demand without inhibitors (versus €1,806 for their matched controls)
€76,332 for patients treated on demand with inhibitors (versus €2,288 for their matched controls)
€247,937 for patients treated in prophylaxis without inhibitors (versus €1,159 for their matched controls)
€349,616 for patients treated in prophylaxis with inhibitors (versus €1,342 for their matched controls).
The majority of the costs was related to antihemophilic drugs: 64%, 60%, 94% and 93% respectively in the 4 treatment groups.
Conclusion:
These results highlight the burden of hemophilia A in terms of comorbidities, healthcare consumptions and cost. The cost of Hemophilia A varied greatly with disease severity and presence of inhibitors and was mostly due to the antihemophilic drugs.
Disclosures
Lebreton:Pfizer: Consultancy; Pfizer: Research Funding. Cahoreau:Pfizer: Consultancy. Delienne:Pfizer: Consultancy. Rudant:Pfizer: Current Employment. Reynaud:Pfizer: Current Employment. Coumert:Pfizer: Current Employment. Lilliu:Pfizer: Consultancy. Frenzel:CSL Berhing: Consultancy, Research Funding; Biomarin: Consultancy; Pfizer: Consultancy, Other: Grant, Research Funding; Roche: Consultancy.