Background: CLL tx options have recently expanded to include second generation (gen) Bruton tyrosine kinase inhibitors (BTKi) and b-cell 2 inhibitors (BCL2i). Current guidelines recommended that these txs be used in 1L CLL tx. We sought to understand how the standard of care has evolved among community oncologists since 2020 and assess which clinical and demographic factors impact tx selection.
Methods: Using the Integra Connect PrecisionQ real-world de-identified database of over 3 million cancer patients (pts) across 500 sites of care, we assessed 1L tx of 6,328 CLL pts between 1/1/2020 and 6/30/2023. For 1L CLL pts to be included, they must have had either >=5 CLL or small lymphocytic lymphoma (SLL) diagnosed visits, or more CLL or SLL visits than non-CLL or SLL visits if <5 CLL or SLL diagnosed visits. We measured 1L tx use of 1 st gen BTKi, 2 nd gen BTKi, BCL2i, anti-CD20 (aCD20), and chemotherapy (chemo). We also assessed how use might be impacted by gender, age, race, ECOG, testing for del17p and TP53, positivity for TP53 or del17p, and pre-existing cardiac conditions. A subset of pts was evaluated through manual chart review to assess for TP53 and del17p mutations (N = 1029). Descriptive analyses were used and proportions were compared using a two-tailed two sample z-test.
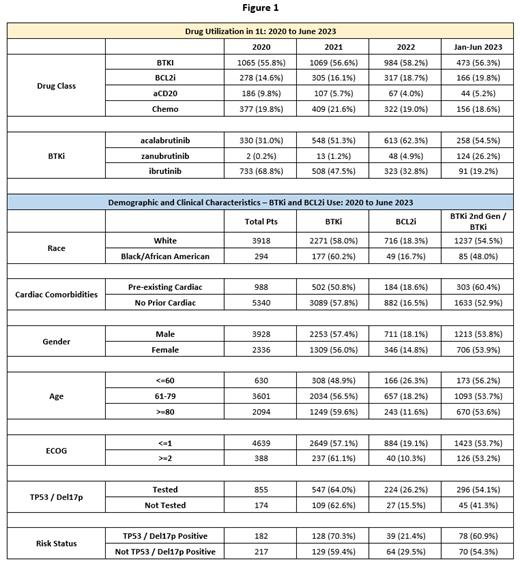
Results: Among the 6,328 CLL pts, the average age was 74.0 (median = 75), the gender distribution was 62.7% male, and 92.3% of pts had an ECOG of 0 or 1 at 1L. In calendar year 2020 (N = 1907), BTKi therapy accounted for 55.8% of 1L therapy, BCL2i accounted for 14.6%, aCD20 accounted for 9.8%, and chemo accounted for 19.8%. In 2020, the percentage of 1L pts treated with zanubrutinib was 0.2%, while acalabrutinib was 17.3% and ibrutinib was 38.4%; among BTKis, 2 nd gen BTKi use was 31.2%. In calendar year 2022 (N = 1691), BTKi therapy accounted for 58.2% of 1L therapy, BCL2i accounted for 18.7%, aCD20 accounted for 4.0%, and chemo accounted for 19.0%, while 2 nd gen BTKi use grew to 67.2% of total BTKi use. For January to June 2023 (N = 840), BTKi therapy accounted for 56.3% of 1L therapy, BCL2i accounted for 19.8%, aCD20 accounted for 5.2%, and chemo accounted for 18.6%. In 2023, the proportion of 1L pts treated with zanubrutinib was 14.7%, with acalabrutinib was 30.7%, and with ibrutinib was 10.8%; among BTKis, 2 nd gen BTKi use increased to 80.7%.
Since 2020 among pts treated with a BTKi, no significant difference in 1L tx based on race was observed when comparing Black/African American pts (N = 177) treated with 2 nd gen BTKis (48.0%) with white pts (N = 2271) treated with 2 nd gen BTKis (54.5%) (p >.05). Of pts with pre-existing cardiac conditions (N = 988), 50.4% received a BTKi, whereas of those without known pre-existing cardiac conditions, 57.8% received a BTKi (N = 5340) (p <.001). Of pts who received a BTKi, those with pre-existing cardiac conditions received a 2 nd gen BTKi at a rate of 60.4% (N = 502), while of those without pre-existing cardiac conditions (N = 3089), 52.9% received a 2 nd gen BTKi (p <.001). Male pts received a higher rate of BTKi or BLC2i 1L tx (75.5%) than did female pts (70.8%) (p <.001). Pts age 60 and under (N = 630) received venetoclax at a rate of 26.3% compared to 11.6% of those age 80 and older (N = 2094) (p <.001). Pts age 60 and under received a BTKi at a rate of 48.9% compared to 59.6% of pts age 80 and older (p <.001). Among pts with a known ECOG, pts with an ECOG of <=1 (N = 4639) received a BTKi or BCL2i 76.2% of the time, compared to 71.4% of the time for pts with an ECOG of >=2 (N=388) (p <0.05). Among those pts evaluated via medical chart review, pts who had been tested for either del17p or TP53 (N = 855) received a BTKi or BCL2i 90.2% of the time, compared to 78.2% of those not tested (N = 174) (p < .001). Among pts with del17p or a TP53 mutation (N= 182), 70.3% received a BTKi, compared to 59.4% of those without del17p or a TP53 mutation (N = 217) (p <0.05).
Conclusion: The combined use of BCL2i and 2 nd gen BTKis in 1L grew from 32.5% in 2020 to 65.2% in 2023 (2 nd gen BTKi use among BTKis increased from 31.2% to 80.7%). However, in 2023, more than 33% of pts did not receive either of the recommended regimens per the guidelines; nearly 20% continue to receive chemo. We found that female pts, pts age 80 or older, pts with ECOG of >=2, and pts without testing for del17p and TP53 received BTKi therapy and BCL2i therapy less often. This suggests a need to increase awareness of the clinical guidelines for tx of CLL and further understand why such differences exist in the tx of CLL pts.
Disclosures
Hou:AbbVie: Consultancy; AstraZeneca: Consultancy; Genentech: Consultancy. Gart:Integra Connect: Current Employment. Vasudevan:IntegraConnect, Precision Q: Current Employment. Katzen:Integra Connect: Current Employment. Choksi:Integra Connect: Consultancy, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings and/or travel . Blanc:IntegraConnect, Precision Q: Current Employment.