Introduction: Global arginine bioavailability ratio (GABR) (Arginine/[Citrulline+Ornithine]) is associated with clinical outcomes including pain, pulmonary hypertension, and mortality in patients with sickle cell disease (SCD). Arginine replacement therapy has demonstrated opioid-sparing effects, improved blood pressure and cardiovascular function, and shorten length of hospital stay. Arginine is a conditionally essential amino acid synthesized from citrulline in the kidneys. During a vaso-occlusive pain episode (VOE), hemolysis leads to the release of erythrocyte-arginase (arginine-metabolizing enzyme) that metabolizes arginine into ornithine. Arginine-to-ornithine ratio (arg/orn) is a biomarker of arginase activity and GABR incorporates the impact of kidney function on global arginine bioavailability. The association between arginine bioavailability and clinical outcomes has not been sufficiently explored in children with SCD-VOE.
Objective: To evaluate associations between arginine bioavailability and clinical outcomes in children with SCD-VOE who received intravenous (IV) arginine replacement therapy.
Methods: We conducted a secondary analysis of a prospective, single-center, double-blinded randomized controlled trial of IV arginine therapy (TID, up to 7 days) in children with SCD age 3-21 years hospitalized for VOE requiring IV opioids. Patients with significant liver/renal dysfunction or those previously enrolled were excluded. Subjects were randomized into 1 of 3 arms: 1) 100 mg/kg/dose arginine (standard dose), 2) loading dose: 200 mg/kg followed by standard dose or 3) placebo. Demographics, total parenteral opioid (TPO) use (morphine equivalents, mg/kg) time to crisis resolution (time of study drug delivery to last IV opioid in hours), pain scores, and targeted amino acids were obtained before treatment and at discharge. Mean±SD, paired t-tests, and Pearson correlation analyses between groups were performed. This trial was registered at www.clinicaltrials.goc as #NCT02536170
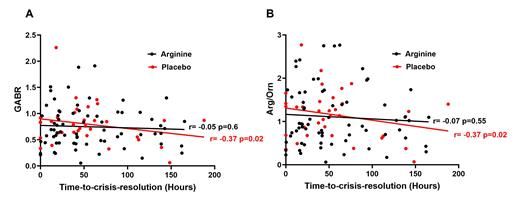
Results: 1,548 patients were screened, 266 were eligible, 114 consented, and 108 were randomized (54.4% were female, mean age 12.44 ± 3.90). All participants identified as African American and 75% had genotype Hb-SS. 69.1% were on hydroxyurea. Safety results of this RCT have been previously reported ( Reyes et al, Am J Hematol 2022). Plasma arginine levels were low at VOE presentation (mean 50±28 μM), with low arginine levels (<60 μM) found in 74% of patients. Arginine replacement therapy increased arginine concentration by 182% (p<0.0001) compared to 26% (p=0.24) in placebo. Arg/orn correlated to arginase concentration ( r= - 0.38, p<0.0001). Time-to-crisis-resolution strongly correlated with TPO ( r=0.72, p<0.0001). Arginine bioavailability at presentation was inversely correlated to time-to-crisis-resolution for both arg/orn and GABR ( r= -0.37 p=0.02) in the placebo arm only but not in those participants who received arginine therapy (GABR: r= - 0.05, p=0.6; arg/orn: r= - 0.07, p=0.55) (Fig1A,1B). Similar trends were observed for TPO. Pain scores at presentation positively correlated with TPO ( r= 0.33, p=0.04) in the placebo arm only, but not in the arm that received arginine therapy ( r= 0.03, p=0.76). Time-to-crisis-resolution positively correlated with age ( r= 0.2, p=0.03).
Conclusion: In the absence of arginine replacement therapy, low arginine bioavailability predicted longer time-to-crisis resolution and a higher use of IV opioids in patients with SCD-VOE. Arginine bioavailability may represent a novel biomarker of SCD-pain severity. This data further confirms arg/orn as a surrogate for arginase activity reflective of hemolytic rate as previously described. Arginine replacement therapy ameliorates the influence of arginine deficiency on clinical outcomes related to pain in SCD. An NHLBI/ Pediatric Emergency Care Applied Research Network (PECARN) supported Phase-3 multi-center trial of arginine replacement therapy enrolling 360 children with SCD (SCD Treatment with Arginine Therapy - STArT) is ongoing.
OffLabel Disclosure:
Morris:UCSF-Benioff Children's Hospital Oakland: Consultancy, Patents & Royalties: Inventor of licensed patents generating royalties; CSL Behring: Consultancy, Other: ad hoc consultant; Roche: Consultancy, Other: ad hoc consultant; Trility: Consultancy, Membership on an entity's Board of Directors or advisory committees; Food as Medicine Therapeutics: Current Employment, Current equity holder in private company, Other: Executive Director of new Start-up company.
L-Arginine Sickle Cell Disease