Background: The standard of care for frontline therapy of acute leukemias of ambiguous lineage (ALAL) remains controversial due to their rarity; however, outcomes are generally poor. Intensive chemotherapy (IC) regimens are standard, with evidence suggesting that acute lymphoblastic leukemia regimens are more effective than those used for acute myeloid leukemia (AML). Strategies for treating older or frail patients are limited. Although treatment with a hypomethylating agent plus venetoclax (HMA/Ven) is standard therapy for IC-ineligible AML patients, the role of HMA/Ven in ALAL remains poorly characterized. We performed a retrospective chart review of patients at our institution with Philadelphia-negative ALAL, comparing the outcomes of those treated with HMA/Ven and those who received IC.
Method: Patients ≥ 18 years treated at Weill Cornell Medical Center with newly diagnosed, pathologically confirmed acute undifferentiated leukemia (AUL), T/myeloid, or Philadelphia chromosome-negative B- and B/T-myeloid leukemia were included. Frontline HMA/Ven was defined as Ven plus azacytydine, decitabine, or oral decitabine/cedazuridine (one patient had initial HMA monotherapy followed by HMA/Ven). Intensive chemotherapy included patients who received variations of standard ALL or AML IC regimens (hyper- or mini-hyper-CV[A]D, cytarabine plus daunorubicin or idarubicin, or cladribine plus idarubicin and cytarabine) with or without Ven. Tyrosine kinase inhibitors including midostaurin or gilteritinib were added for patients with fms-like tyrosine kinase 3 ( FLT3) mutations. Baseline and disease characteristics were collected as well as disease response, subsequent lines of therapy, whether the patient proceeded to allogeneic hematopoietic cell transplantation (HCT), rate of morphologic complete remission (CR), and overall survival (OS).
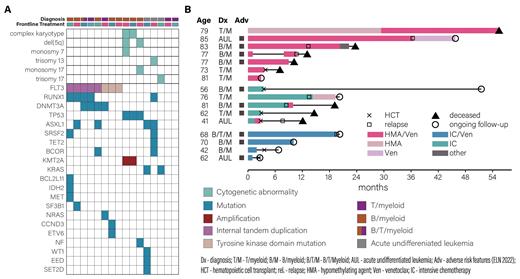
Results: Of 16 patients, 7 received frontline HMA/Ven. B/myeloid was the most common disease type (n=8), followed by T/myeloid (n=4), AUL (n=3), and 1 patient with B/T/myeloid disease. The median age was 74 years (interquartile range [IQR] 62-80). Patients who received HMA/Ven were older than those who received IC, with median ages of 79 (IQR: 77-82) and 62 (IQR: 56-70; p = 0.01), respectively. Baseline white blood cell, hemoglobin, platelet counts, and lactate dehydrogenase, as well as blast percentages in both peripheral blood and bone marrow were similar between the groups. Although not validated in ALAL, markers of adverse myeloid disease as per the ELN 2022 schema were prevalent in both groups, including mutations in RUNX1, ASXL1, SRSF2, and TP53 (Figure A). One HMA/Ven patient and 3 IC patients underwent HCT. Of evaluable patients, 83% and 88% of HMA/Ven and IC patients achieved CRs, respectively. At 2 years, both groups had 50% overall survival (95% CI 22.5-100 in both). Among HMA/Ven recipients, overall survival ranged from 10-56 months, not including two patients with ongoing follow-up at 3 and 46 months (Figure B).
Discussion: The treatment of ALAL remains a challenge, particularly in older patients who may be unfit for intensive treatment strategies. In our study, patients who received HMA/Ven had similarly durable disease control to younger patients who received IC in the frontline setting. Prolonged responses were seen in both AUL and MPAL. Multicenter registry and prospective analyses are needed; however, given the rarity of these diagnoses, our findings suggest that HMA/Ven should be considered as a frontline therapy for unfit patients with ALAL.
Disclosures
Bar-Natan:Amgen: Research Funding; BMS: Research Funding; Incyte: Research Funding. Chadburn:Medical College of Wisconsin: Honoraria; Boehringer Ingelheim Pharmaceuticals, Inc.: Consultancy; Leica Biosystems: Consultancy. Desai:Janssen Pharmaceuticals: Current Employment; Servier: Consultancy, Other: Advisory role; Abbvie: Consultancy, Other: Advisory role; BMS: Consultancy, Other: Advisory role; Janssen Research & Development: Research Funding. Ritchie:Celgene, Incyte Corporation, Novartis: Consultancy; Pfizer: Consultancy, Other: travel, Research Funding; Celgene: Other: Travel Support, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding; Astellas Pharma, Jazz Pharmaceuticals, NS Pharma: Research Funding; Ariad: Speakers Bureau; Novartis: Consultancy, Other: Travel Support, Research Funding, Speakers Bureau. Roboz:Celgene/Bristol Myers Squibb: Consultancy; Amgen: Consultancy; Abbvie: Consultancy; Argenx: Consultancy; Astra Zeneca: Consultancy; Blueprint Medicines: Consultancy; Bluebird Bio: Consultancy; Daiichi Sankyo: Consultancy; Ellipsis Pharma: Consultancy; Glaxo Smith Kline: Consultancy; Janssen: Consultancy, Research Funding; Jasper pharmaceuticals: Consultancy; Jazz Pharmaceuticals: Consultancy; Molecular Partners: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Roche: Consultancy; Takeda: Consultancy; Syndax: Consultancy.