CD37 is a trans-membrane protein specifically expressed on normal hematopoietic tissues such as B cells, neutrophils and macrophages, and has demonstrated roles in adhesion, cell migration (de Winde et al, 2018; Wee et al, 2015), survival and apoptotic signals (Lapalombella et al, 2012). Increased expression of CD37 has been observed in various hematological cancers such as lymphomas, Chronic Lymphocytic Leukemia (CLL) and Acute Myeloid Leukemia (AML) (Beckwith et al, 2014; Deckert et al, 2013; Pereira et al, 2015; Yan et al, 2021). Higher expression of CD37 was also associated with poor patient outcome in AML (Zhang et al, 2020; Larkin et al, ASH 2018).
Debio 1562M is a second-generation novel ADC directed against CD37 and including Debiopharm's proprietary Multilink cleavable linker-payload technology. Multilink improves manufacturing to achieve homogenous high drug antibody ratio while retaining excellent solubility as well as favorable biologic characteristics with preserved internalization kinetics on target cells. Debio 1562M is conjugated to 8 molecules of a tubulin-binding agent and despite cleavable linker that ensure effective intracellular delivery of toxic payload, we show sustained plasma stability in vitro and in vivo with no premature extracellular release.
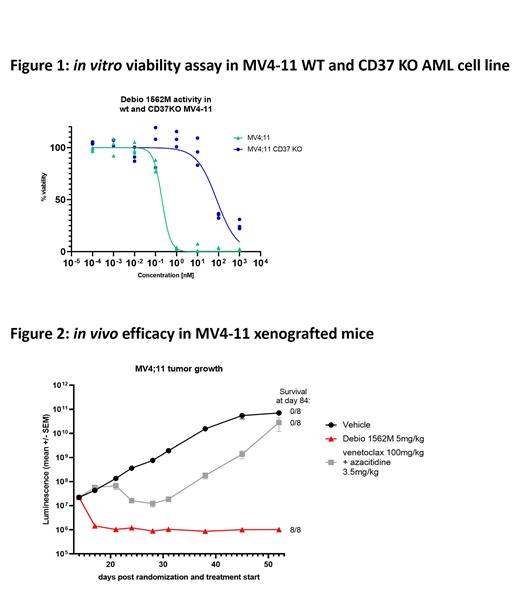
In this work, we report Debio 1562M specificity and potent antitumor activity in multiple AML models both in vitro and in vivo. First, in our in vitro cytotoxicity assay, Debio 1562M displayed nano- to picomolar anti-proliferative activity in both AML cell lines and primary samples. Out of 30 primary samples tested, 20 were sensitive to Debio 1562M with IC50 ranging from 1 to 10nM, this regardless of their mutation status, subtype or medical history. Interestingly, we observed that AML cells have high internalization capability and therefore relatively low levels of surface CD37 is sufficient for cytotoxic activity. The specificity of Debio 1562M was demonstrated by using 3 different CD37 knock out AML cell lines which were generated by CRISPR-Cas9 technology. Parental and CD37 KO cells were treated for 72h with a dose range of Debio 1562M and viability was measured (Figure 1). While Debio 1562M has an IC50 below 1nM in all 3 parental cell lines, its activity is reduced by more than 2 log in the KO cell lines. In vivo, in 3 different AML cell line xenograft mouse models, a single injection of Debio 1562M at 5mg/kg induced complete tumor regression and all mice remained alive at study termination, more than 60 days after treatment, except one mouse in one model. In a patient derived xenograft (PDX) model, the same dose of Debio 1562M significantly reduced tumor burden in blood and bone marrow with sustained activity 42 days after treatment (37% and 98% of blasts in blood and bone marrow respectively in control animals versus 0% and 3% in treated mice, pvalue<0.001). Furthermore, we evaluated the activity of Debio 1562M over venetoclax and azacytidine combination in the MV4-11 Luciferase xenografted mouse model. NCG mice were inoculated with 1x10 7 cells in the tail vein and randomization in groups of 8 mice was performed 14 days later. Mice received either one administration of Debio 1562M at 5mg/kg or a combination of venetoclax (QDx14 100mg/kg) and azacitidine (QDx5 3,5mg/kg). Complete regression of the tumor was observed in all mice after Debio 1562M administration and all mice survived at study termination, 75 days after treatment while none survived after 56 days when treated with venetoclax and azacitidine combination (Figure 2).
The toxicology profile of Debio 1562M in mice showed expected clinical signs related to the payload's off target toxicity but with a significant safety margin. Interestingly, no peripheral neuropathy symptoms were detected, as commonly observed for this class of payload. Additionally, Multilink TM-payload construct alone was not associated with any signs of toxicity. On-target toxicity profile couldn't be evaluated as Debio 1562M isn't cross reactive with mouse CD37, however 1 st generation CD37 ADC has already demonstrated safety and tolerability in Phase 1 and 2 clinical trials.Overall, our data confirm the relevance of CD37 targeting for AML treatment. The excellent antitumor activity and tolerability of Debio 1562M in several pre-clinical models now pave the way for upcoming clinical development.
Disclosures
Ivanschitz:Debiopharm international: Current Employment. Hue-Perron:Debiopharm international: Current Employment. Marx:Debiopharm Research and Manufacturing: Current Employment. Roubaudi-Fraschini:Debiopharm international: Current Employment. Peet:Debiopharm international: Current Employment. Bellocq:Debiopharm Research and Manufacturing: Current Employment. Colombo:Debiopharm international: Current Employment. Larkin:Gilead: Honoraria; Debiopharm international: Research Funding; Astellas Pharma: Consultancy.