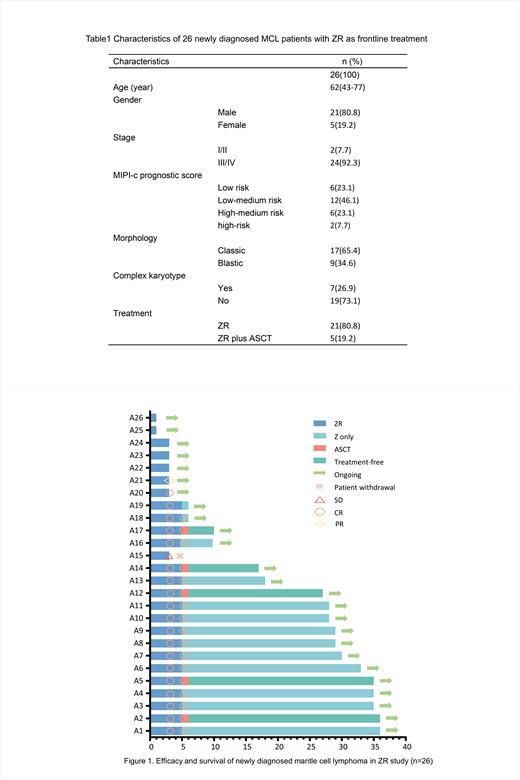
Background:Mantle cell lymphoma (MCL) is an incurable B-cell lymphoma with heterogeneous clinical presentation. Frontline intensive chemoimmunotherapy induction with or without autologous stem cell transplantation (ASCT) as consolidation is recommended. However, this approach has the tendency to increase short- and long-term toxicities. Window-1 study demonstrated upfront use of ibrutinib-rituximab induction allowed minimization of chemotherapy exposure, thereby reducing adverse events. Based on favorable response rate and superior safety of zanubrutinib in relapsed/refractory (R/R) MCL patients, combination of zanubrutinib plus rituximab (ZR) might obviate the need for chemotherapy in newly diagnosed MCL patients.
Aims:This study aimed to analyze the efficacy and safety of ZR chemo-free therapy with or without ASCT in newly diagnosed MCL patients.
Methods:Patients with newly diagnosed MCL were enrolled in this prospective, open-label phase II study (NCT05504603). Patients received oral zanubrutinib(160 mg twice daily) and rituximab (375 mg/m 2 weekly for cycle 1 and then on day 1 of cycles 2-5, 4 weeks per cycle) as induction therapy. Then, patients who achieved complete remission (CR) or partial remission (PR) were followed by zanubrutinib maintenance for elderly (age >70y) or unfit patients until intolerable toxicities or relapsed, while ASCT was given to younger (age ≤70y) and fit patients. The primary endpoints were overall response rate (ORR) and complete response rate (CRR) at the end of cycle 3, cycle 5 and ASCT. The secondary endpoints were 2-year overall survival (OS), 2-year progression free survival (PFS) and safety profiles.
Results: Twenty-six patients with a median age of 62 years were enrolled. The majority of patients had intermediate-risk international prognostic index and stage III/IV disease. Next-generation sequencing was performed in 21 patients to detect genetic mutations including ATM (n=9, 42.9%) and TP53 (n=3, 14.3%) mutations. 21 patients completed at least 3 cycles of ZR with 19 patients achieved CR, 1 got PR and 1 got stable disease who was then taken off this trial. The ORR and CRR for a 3-cycle response were 95.5% and 90.9%, respectively. 17 patients completed the planned 5-cycle treatment with 5 patients proceeding to ASCT. Among the 17 patients, all patients achieved CR with 10 patients (58.8%) achieved minimal residue disease negative. Within a median follow-up of 18.7 months, the 2-year OS and PFS were both 100%, and the median OS and PFS were undefined. During the whole treatment, 5 cases experienced transient alanine transaminase elevation, oral mucositis and hematological toxicities, 4 cases showed pyrexia, 2 cases occurred infusion-related response, and 1 case occurred COVID-19-related pneumonia. All toxicities were transient and reversible. No atrial fibrillation and bleeding were observed.
Conclusions: This study demonstrates a favorable efficacy and tolerable safety of chemo-free regimen ZR in newly diagnosed MCL patients and expands therapeutic options for newly diagnosed MCL.
Keywords: Mantle cell lymphoma; Zanubrutinib; Rituximab; Autologous stem cell transplantation; Therapy.
Disclosures
No relevant conflicts of interest to declare.