Introduction: Zanubrutinib is a potent and highly selective next-generation Bruton tyrosine kinase (BTK) inhibitor designed to maximize BTK occupancy and minimize off-target effects. In the phase 2 ROSEWOOD study (NCT03332017), zanubrutinib plus obinutuzumab (ZO) demonstrated superior efficacy and a manageable safety profile compared with obinutuzumab monotherapy (O) as treatment for patients with heavily pretreated R/R FL. Here, we report HRQoL outcomes from the ROSEWOOD trial.
Methods: Patient-reported outcomes (PROs) were secondary endpoints and assessed using the European Organisation for Research and Treatment of Cancer Quality of Life of Cancer Patients Questionnaire - C30 (EORTC QLQ-C30) and European Quality of Life 5-Dimensions 5-Levels (EQ-5D-5L). Patients completed questionnaires at baseline (cycle 1 day 1, before the first dose of study drug), then every 12 weeks for 2 years, every 24 weeks for the next 2 years, and then annually until disease progression, death, or withdrawal of consent. Compliance rates were calculated as the number of patients who completed questionnaires vs. the number expected to complete questionnaires at each visit in each arm. PRO endpoints included the EORTC QLQ-C30 global health status (GHS), physical and role function domains, and fatigue, pain, nausea/vomiting, and diarrhea symptom domains, assessed via a mixed model for repeated measures (MMRM) analysis at weeks 12 and 24. Clinically meaningful change was defined as ≥5-point mean difference from baseline (Osoba et al., J Clin Oncol 1998). Descriptive analyses, including change over time, were also performed on QLQ-C30 domains and EQ-5D-5L visual analog scale (VAS) scores.
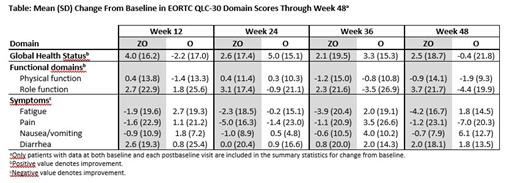
Results: A total of 217 patients were randomized to ZO (n=145) and O (n=72), with similar baseline demographics and disease characteristics between the arms. The median (range) duration of study treatment was 12.2 (0.5-44.1) and 10.6 (0.3-30.3) months, respectively. Compliance rates for PRO assessments in both arms were over 80% at week 12, and 85% at week 24. By week 24, patients in the ZO arm had better overall outcomes in the QLQ-C30 domains of role function, pain, and fatigue than those in the O arm, while patients in both arms experienced improvement in GHS, and physical function and symptoms of nausea/vomiting and diarrhea were maintained ( Table). MMRM analyses revealed clinically meaningful improvement from baseline to week 24 for ZO vs O in change in role function (least squares mean difference [95% CI]: 5.6 [-2.3, 13.5]), pain (-4.9 [-12.6, 2.8] points), and fatigue (-4.7 [-11.6, 2.2]). Based on a descriptive analysis, patients in the ZO arm also experienced greater mean improvement from baseline to week 24 vs the O arm in EQ-5D-5L VAS scores (3.1 vs 2.0).
Conclusions: In the ROSEWOOD trial, ZO was associated with improved HRQoL in patients with R/R FL. In particular, patients who received the ZO had larger improvements in fatigue, pain symptoms, and role function, than those who received O monotherapy. These findings suggest that zanubrutinib contributed clinically meaningful benefits to patient HRQoL when added to obinutuzumab.
Disclosures
Trotman:BMS: Research Funding; Cellectar: Research Funding; Janssen: Research Funding; Roche: Research Funding; BeiGene: Research Funding. Zinzani:ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees; SANDOZ: Membership on an entity's Board of Directors or advisory committees; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Delarue:BeiGene: Current Employment; Celgene/Bristol Myers Squibb, BeiGene: Current holder of stock options in a privately-held company. Barnes:BeiGene: Current Employment, Current holder of stock options in a privately-held company. Kim:BeiGene: Current Employment, Current holder of stock options in a privately-held company. Ivanova:BeiGene: Current Employment, Current holder of stock options in a privately-held company. Tang:BeiGene: Current Employment. Mayer:MSD: Research Funding; BeiGene: Research Funding. De Oliveira:Janssen: Other: Travel Expenses; Janssen, Alexion: Consultancy. Assouline:Roche-Genentech: Honoraria; AstraZeneca: Honoraria; BeiGene: Consultancy; Novartis Canada: Research Funding; AbbVie: Honoraria; Janssen: Honoraria; Ipsen: Consultancy; Gilead: Honoraria; Palladin: Honoraria. Flowers:Burroghs Wellcome Fund: Research Funding; Ziopharm: Research Funding; Xencor: Research Funding; TG Therapeutics: Research Funding; Takeda: Research Funding; Sanofi: Research Funding; Pharmacyclics: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Nektar: Research Funding; Morphosys: Research Funding; Kite: Research Funding; Jannsen Pharmaceuticals: Research Funding; Iovance: Research Funding; Guardant: Research Funding; Cellectis: Research Funding; Amgen: Research Funding; Allogene: Research Funding; Adaptimmune: Research Funding; Acerta: Research Funding; 4D: Research Funding; Spectrum: Consultancy; SeaGen: Consultancy; Pharmacyclics Jansen: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Karyopharm: Consultancy; Gilead: Consultancy, Research Funding; Genmab: Consultancy; Genentech Roche: Consultancy, Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Denovo Biopharma: Consultancy; Celgene: Consultancy, Research Funding; Beigene: Consultancy; Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; Eastern Cooperative Oncology Group: Research Funding; V Foundation: Research Funding; National Cancer Institute: Research Funding; CPRIT Scholar in Cancer Research: Research Funding.