There is no universal approach to treat patients with relapsed and refractory (RR) mature T-cell and NK-cell neoplasms (TNKL). While participation in a clinical trial is favored, appropriate options are often unavailable. Thus, outside of a trial, physicians may rely on real world evidence when making a treatment choice. We hypothesized that innovative causal predictive models that utilize existing clinical information could estimate comparative efficacy of various treatments and support clinical decision making.
As the first step, we built a large global cohort of 925 patients with RR TNKL diagnosed between Jan 2010 and Dec 2020 representing 9 countries. Eligible patients received either a single agent (SA) such as an epigenetic modifier, small molecule inhibitor, brentuximab vedotin (BV) among others, or platinum-, gemcitabine-, ifosfamide-based cytotoxic chemotherapy (CC) in the second line setting. Patients were followed from lymphoma diagnosis, or first retreatment start date to death or lost to follow-up. Thirteen covariates [relapsed/refractory state, with refractory defined as lack of complete remission (CR) to first line therapy, country Japan, age >50 years, histological subtype AITL, IPI, PIT score, response to 1 st line treatment, 2 nd line treatment (SA or CC), response to 2 nd line treatment, autologous stem cell transplantation (Auto-SCT), response to ASCT, allogeneic stem cell transplantation (Allo-SCT), response to Allo-SCT] were identified to have an independent prognostic effect on overall survival (OS). Based on measures of Brier score (BS) and Concordance index (CI), eight of them were selected for multivariate Cox regression model. We observed comparable performance of the Cox models utilizing these 8 covariates (CI: 0.755; BS:0.174) versus 5 of these 8 covariates (CI:0.744; BS:0.186). Hence 5 covariates were utilized for further OS analyses.
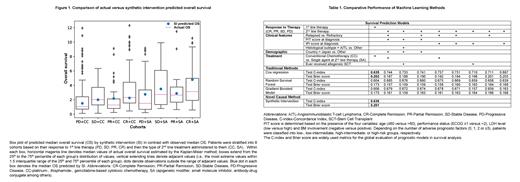
We found that multivariate Cox outperforms other traditional models including random survival forest and gradient boosted (Table 1). Since data under consideration is observational and not derived from a clinical trial, there may be several (unobserved) confounders leading to bias in the outcomes, which are not adjusted for by these traditional approaches. We introduce a novel causal method, Synthetic Intervention (SI), which adjusts for such biases. For comparative evaluation of SI with multivariate Cox, due to data requirement for SI, we chose only two covariates [response to 1 st line stratified as CR, partial remission (PR), stable disease (SD) and progressive disease (PD) and administration of second line treatment (SA or CC)] (Table 1). We found that SI outperforms multivariate Cox. Further, despite heterogeneity in OS across the 8 cohorts, the median OS prediction of SI was comparable to actual median OS for each cohort, thus highlighting robustness of SI (Figure 1). All the performance evaluations were done using 60:40 train: test split, repeated 500 times.
We confirmed previous reports that relapsed patients had superior OS compared to primary refractory patients across common histologies: PTCL-NOS (p<.0001), AITL (p=.036), ENKTCL (p=.027), and ALK- ALCL (p=.001). Those who received SA at first retreatment had superior OS compared to those treated with CC in AITL (p<.0005) and ALK- ALCL (p=.036) and comparable OS in PTCL-NOS, ENKTCL, and ALK+ ALCL. In ALK- ALCL, those treated with BV had superior OS compared to CC (p=.027). Patients with AITL treated with small molecule inhibitors (SMI) had greater OS compared to those treated with CC (p<.0005) and epigenetic modifiers (EM) (p=.001), with the most common SMIs including alisertib, duvelusib, cerdulatinib, and cyclosporine. Those who responded to EM and BV (CR+PR+SD) had superior OS compared to non-responders (PD) in PTCL-NOS (p=.004) and ALK- ALCL (p<0.0005). Among patients who underwent SCT after second line therapy, those bridged with SA had comparable OS to CC in PTCL-NOS and an improved trend (p=.073) in AITL.
In summary, we have characterized the largest global cohort of RR patients with TNKL in the era of contemporary therapies. As a causal analysis method, SI demonstrates the causal effect superiority of SA over CC in terms of survival outcomes for patients who responded differently to their 1st line treatment. Moreover, it achieves prediction accuracy comparable to, if not better than, Cox regressions, one of the most widely used methods in survival analysis.
OffLabel Disclosure:
Chiattone:ROCHE, ABBVIE, JANSSEN, AZ, LYLLI, TAKEDA: Honoraria; ROCHE, ABBVIE, JANSSEN, AZ, LYLLI, TAKEDA: Consultancy. Horwitz:ONO Pharmaceuticals: Consultancy; Affimed: Research Funding; Tubulis: Consultancy; Abcuro Inc.: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Celgene: Research Funding; Cimieo Therapeutics: Consultancy; Auxilius Pharma: Consultancy; Trillium Therapeutics: Consultancy, Research Funding; Kyowa Hakko Kirin: Consultancy, Research Funding; SecuraBio: Consultancy; Shoreline Biosciences, Inc.: Consultancy; Takeda: Consultancy, Research Funding; Yingli Pharma Limited: Consultancy; ADC Therapeutics: Research Funding; Millenium: Research Funding; Crispr Therapeutics: Research Funding; Seattle Genetics: Research Funding; Verastem/SecuraBio: Research Funding. Jacobsen:Celgene: Research Funding; Merck: Honoraria, Research Funding; Pharmacyclics: Research Funding; Hoffman-LaRoche: Research Funding; Daiichi: Honoraria; BMS: Honoraria; Bayer: Honoraria; UpToDate: Patents & Royalties. Jain:ImmunoACT: Research Funding; Zydus Pharmaceuticals: Research Funding; Intas Pharmaceuticals: Research Funding. Van Der Weyden:Cartherics Pty Ltd: Ended employment in the past 24 months, Membership on an entity's Board of Directors or advisory committees. Prince:Takeda: Speakers Bureau; Merck: Speakers Bureau; Mallinkrodt: Speakers Bureau; Mundipharma: Speakers Bureau. Foss:SecuraBio: Honoraria; Daiichi Sankyo: Honoraria; Kyowa: Honoraria; Conjupro: Honoraria; Astex: Honoraria; Seagen: Speakers Bureau; Acrotech: Speakers Bureau. Casadei:Kite-Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Speakers Bureau; Lilly: Speakers Bureau; Roche: Speakers Bureau; Celgene-BMS: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Zinzani:SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kim:Sanofi, Beigene, Boryong, Roche, Kyowa-kirin, Donga: Research Funding. Verburgh:MSD: Research Funding. Shen:Biogen Digital Health: Current Employment. Marchi:Merck: Research Funding; Celgene/BMS: Research Funding; Astex Pharmaceutical/Myeloid Pharmaceuticals: Research Funding; Dren Bio: Membership on an entity's Board of Directors or advisory committees, Research Funding; Everest Clinical Research: Other: Data Safety Monitoring Committee. Jain:Mersana Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; SecuraBio: Membership on an entity's Board of Directors or advisory committees; SIRPant Immunotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abcuro, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees, Research Funding; Crispr Therapeutics: Membership on an entity's Board of Directors or advisory committees; Acrotech LLC: Research Funding.
There is no established standard of care in relapsed or refractory mature T and NK cell neoplasms. Agents reported in this observational study may have been used off-label as part of clinical practice.