Background: Myelodysplastic syndromes (MDS) are a heterogeneous group of hematologic malignancies characterized by ineffective erythropoiesis. Anemia is the most common cytopenia and major clinical problem in MDS. Acquired pyruvate kinase (PK) deficiency has been observed in MDS, and a direct impact on the glycolytic pathway in the pathogenesis of anemia associated with MDS has been demonstrated. AG-946 is an investigational, small-molecule, allosteric activator of PK that has the potential to enhance red blood cell (RBC) functionality and survival by increasing glycolysis and adenosine triphosphate (ATP) production, and improve differentiation of erythroid cells in bone marrow, potentially improving anemia caused by ineffective erythropoiesis in MDS. Previous findings showed that RBCs from patients with MDS treated ex-vivo with AG-946, led to an increase in PK activity. Here, we used two MDS-related mouse models to evaluate the effects of the PK activator AG-946 in this mechanism of anemia.
Aim: Evaluate the effect of AG-946 treatment on impaired erythropoiesis in the bone marrow of two mouse models of MDS.
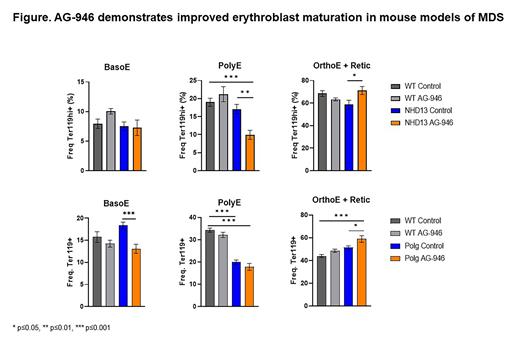
Methods:NUP98-HOXD13 (NHD13) is a fusion gene mutation observed in some patients with MDS. Mouse models with this mutation develop key characteristics of MDS such as impaired erythropoiesis and progressive anemia leading to malignant leukemia around 12 months of age. Male and female NHD13 and C57BL/6J mice were placed on 0.006% AG-946 (approximate dose 10 mg/kg/day) formulated diet starting at 10 months given ad libitum and continuing for 3 months. Numbers of animals for wild type (WT) Control, WT AG-946, NHD13 Control, NHD13 AG-946 were 10, 10, 13, and 11, respectively.Polg D257A (Polg) mouse model, while not a mutation observed in patients with MDS, does display many key features of MDS, such as impaired erythropoiesis and progressive anemia. Male and female Polg mice were placed on 0.006% AG-946 (approximate dose 10 mg/kg/day) formulated diet starting at 4 months given ad libitum and continuing for 8 months. Numbers of animals for WT Control, WT AG-946, Polg Control, Polg AG-946 were 10, 10, 19, and 18, respectively. Bone marrow from 1 femur per mouse was collected and analyzed by flow cytometry. Bone marrow cell pellets were washed and treated with ACK lysing buffer prior to staining. Samples with fewer than 1000 Ter119+ events were removed from analysis due to insufficient cell count. Bone marrow erythroblast populations were gated using Single Cells/Live/B220-/Ter119+/CD44 vs FSC, which were then gated into three populations: basophilic erythroblasts (BasoE), polychromatic erythroblasts (PolyE), and orthochromatic erythroblasts and reticulocytes (OrthoE + Retic).
Results:No differences in any erythroblast populations were observed between untreated WT and NHD13 mice. No changes in the BasoE population were observed in WT or NHD13 mice. The PolyE population in NHD13 mice treated with AG-946 was significantly decreased compared with both the NHD13 and WT untreated mice (p=0.0021 and 0.0003, respectively). NHD13 mice treated with AG-946 showed significant increases in the OrthoE + Retic populations over the untreated NHD13 mice (p=0.0275).No differences in BasoE or OrthoE + Retic populations were observed between untreated WT and Polg mice while the PolyE population was significantly decreased in Polg mice compared with WT (p<0.0001). The BasoE population was significantly decreased in Polg mice treated with AG-946 compared with untreated Polg (p=0.0002). No difference in PolyE population was observed in Polg mice with AG-946. OrthoE + Retic population was significantly increased in Polg mice treated with AG-946 compared with WT and Polg untreated mice (p=0.0001 and 0.0304, respectively). Data graphed as average +/- SEM.
Conclusions: Our data suggest AG-946 treatment improves erythroblast maturation in two MDS mouse models as demonstrated by a reduction in early-stage erythroblasts (BasoE, PolyE) coupled with an increase in late-stage erythroblast populations (OrthoE + Retic). These data are the first to suggest that PK activation by AG-946 could improve ineffective erythropoiesis in MDS.
Disclosures
Bunda:Agios Pharmaceuticals, Inc.: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Gao:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Burgwardt:Agios Pharmaceuticals, Inc.: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Ingersoll:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Jamwal:Agios Pharmaceuticals, Inc.: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Dang:Agios Pharmaceuticals, Inc.: Consultancy. Wind-Rotolo:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company.