Introduction:In recent years, many B-cell maturation antigen (BCMA) directed therapies (BDT) have demonstrated clinical response in heavily treated relapsed/refractory multiple myeloma (RRMM). These therapies include antibody-drug conjugates (ADC), chimeric antigen receptors (CAR-T), T-cell engaging bispecific antibodies (BsAb), and BCMA monoclonal antibodies (MoAb). BDT represents a breakthrough in the treatment of heavily treated RRMM. While these agents have led to deep responses and remissions, they have not been curative and many patients are exposed to more than one BDT. Optimal sequencing of BDT and outcomes in patients who received 2 or more BDT is not well understood. Our study aims to address these questions in this population.
Methods: A retrospective review of 39 patients with RRMM that received 2 or more types of BDTs at the University of Kansas Medical Center, in collaboration with the U.S Myeloma Innovations Research Collaborative (USMIRC), as of 7/10/2023 was completed. Responses to therapy including overall response rate (ORR), complete response or better (>CR), and very good partial response (VGPR) were evaluated using the International Myeloma Working Group (IMWG) criteria. The Kaplan-Meier method was used for progression free survival (PFS) and overall survival (OS) assessments. OS was calculated from exposure of 1 st BDT till the last office visit/death. Regression models for univariate and multivariate analysis were performed.
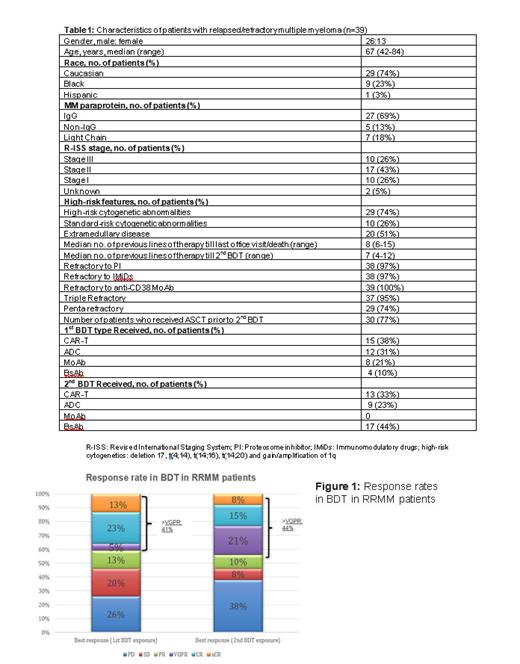
Results: Patients were previously treated with a median of 7 (4-12) prior lines of therapy before 2 nd BDT; 75% were Caucasians, 69% had IgG isotype, 74% had high-risk cytogenetics, and 51% had extramedullary disease (EMD). All patients were triple-class exposed, 95% were triple-class refractory, 82% were penta-class exposed and 74% were penta-class refractory, only 15% received 3 different types of BDTs. Median time between relapse after 1 st BDT to initiation of 2 nd BDT was 4 (0.5-33) months. Baseline characteristics are summarized in Table 1. Median follow-up was 19.4 (11.5-NR) months for the entire cohort.
The most common first BDT were CAR-T and ADC (n=15, 38% and n=12, 31%), respectively. Whilethe most common second BDT were BsAb and CAR-T (n=17, 44% and n=13, 33%), respectively. The ORR after 1 st BDT and 2 nd BDT was 54% and 54%, respectively. The responses after 1 st BDT and 2 nd BDT were found to be deep (41% ≥VGPR, 36% ≥CR) and (44% ≥VGPR; 23% ≥CR), respectively. Detailed responses for the groups of interest are reported separately in Figure 1. The estimated median PFS for 1 st BDT was 5.8 months (95% CI 2.8-9.5) while for 2 nd BDT it was 5.2 months (95% CI 1.77-NA). Median OS from exposure to 1 st BDT was 19.5 months (95% CI 16.7-NA). At the time of data cut-off, 51% of patients had died with 90% of deaths were due to disease progression.
Conclusions: This is one of the first reports on outcomes with second BDT in RRMM. Despite a heavily pre-treated patient population, 2 nd treatment with a BDT resulted in deep and durable responses, similar to treatment with 1 st BDT. Progression free survival was similar with 1 st and 2 nd BDT exposure. While our study is limited by a small population, our results support the use of a different type of BDT at progression even if previously exposed to a BCMA targeting agent. Further studies are needed to provide more guidance on appropriate sequencing of BDTs.
Disclosures
Paul:Janssen: Membership on an entity's Board of Directors or advisory committees. Ahmed:Kite/Gilead: Consultancy, Research Funding; BMS: Other: Ad Board. Mahmoudjafari:Omeros: Speakers Bureau; Pfizer, Genentech, Inc., BMS, KITE, Sanofi, Janssen: Honoraria; Genentech, Inc.: Consultancy.