Background: Topically active corticosteroids have been shown to effectively treat inflammatory bowel disease with a systemic steroid-sparing effect. However, previous studies have not demonstrated their efficacy in preventing graft-versus-host disease (GVHD) when combined with the standard GVHD prophylaxis regimen consisting of calcineurin inhibitors and methotrexate. We hypothesized that the addition of orally administered ileal-controlled-release budesonide to the post-transplant cyclophosphamide (PTCy)-based regimen could effectively prevent severe acute gastrointestinal GVHD. In this study, we present outcomes of the first 43 consecutively treated patients (pts) with the PTCy-tacrolimus (T), mycophenolate mofetil (M) and budesonide (B) (PTCy-TMB) as a GVHD prophylaxis regimen after allogeneic stem cell transplantation (alloSCT).
Methods: Pts with hematologic malignancies received their first alloSCT at our institution between 05/2020-04/2023. Thirty one pts received conditioning with fludarabine (160 mg/m 2) + melphalan (100-140 mg/m 2) + TBI (2-4Gy) or thiotepa 5 mg/kg. Twelve pts received fludarabine (150mg/m 2) + cyclophosphamide (29mg/kg) and TBI 4Gy ± ATG (4.5 mg/kg). GVHD prophylaxis consisted of Cy (50 mg/kg on D+3 and +4), T (trough goal 6-10 ng/mL), M (15mg/kg TID) and budesonide (3 mg TID) starting on D+5 and continued until D+90 followed by a weekly taper if no acute GVHD (aGVHD) occurred. T was continued for at least 6 months followed by a taper, unless otherwise indicated.
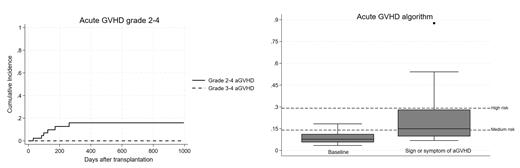
Results: The median age was 50 years (range 22-73), 39 pts had hematologic malignancies including AML (N=14), ALL (N=11) and NHL (N=8). Seven pts (16%) classified as high/very-high DRI. The median of HCT-CI was 4 (range 0-7). Donors were haploidentical (N=32), MSD (N=8), MUD (N=1), MMSD (1) and MMUD (N=1). PBSCs were used in 28 pts (65%). Thirty pts (70%) received RIC regimens. Median follow-up was 407 days (range 91-996 days). At day 100, the cumulative incidence (CI) of grade 2 aGVHD was 7.0% (95%CI 1.8-17.2), while none developed grade 3-4 aGVHD (Figure 1). One patient had severe chronic GVHD (bronchiolitis obliterans) with CI at 2 years of 3.2% (95%CI 0-14.0).
The CI of any systemic steroid use at D+100 and D+180 was 30.2% (95%CI 17.4-44.1) and 44.8% (95%CI 28.9-59.5), respectively. Among the pts who received treatment for aGVHD, the median cumulative systemic steroid exposure (in prednisone equivalents) was 1,318 mg (IQR 623-3,775) at D+100 and 1,325 mg (IQR 525-1,640) at D+180, with a median treatment duration of 40 days (IQR 25-110).
At 1 year, the OS, PFS, GRFS, relapse and NRM were 79.6% (95%CI 61.4-89.9), 69.8% (95%CI 51.6-82.3), 66.6% (95%CI 48.4-79.8), 15.3% (95%CI 6.2-28.2) and 14.9% (95%CI 5.3-29.1), respectively. The 1-year CI of newly diagnosed/worsening hyperglycemia and Cushing syndrome was 18.2% (95%CI 7.9-31.9) and 5.0% (95%CI 0.9-14.2), respectively. 3 pts (7%) required insulin treatment.
The median absolute lymphocyte count (ALC) at day 30, 90 and 180 was 200/mcL, 300/mcL and 500/mcL, respectively, with no differences in the median ALC at D+180 among pts with and without GVHD. Median cell numbers of CD3 +, CD3 +/CD4 +, CD3 +/CD8 +, CD19 + and CD56 + at 1, 3, 6 months post-transplant were D+30 - 63/mcL, 18/mcL, 34/mcL, 1/mcL, 87/mcL, D+90 - 111/mcL, 64/mcL, 34/mcL, 9/mcL, 216/mcL and D+180 - 269/mcL , 78/mcL , 126/mcL , 7/mcL, 189/mcL, respectively.
After 10/2022 pts were tested routinely for Reg3α and ST2 (baseline and retested if developed any signs/symptoms of aGVHD). Among 18 tested pts at baseline, the median number (range) of Reg3α and ST2 at baseline was 28 ng/mL (16-90) and 23,308 pg/mL (4,678-56,226), respectively. Three low-risk pts developed grade 1 aGVHD (N=2) and grade 2 aGVHD (N=1). Fourteen pts with GI sign/symptoms after transplant showed median number (range) of Reg3α and ST2 of 78 ng/mL (16-837) and 34,904 pg/mL (13,329-160,000), respectively. Only one pt with high-risk aGVHD algorithm developed grade 2 aGVHD (Figure 1).
Conclusions: The addition of budesonide to PTCy-based GVHD prophylaxis regimen (PTCy-TMB) is feasible and appears to be effective in preventing severe GI aGVHD, without compromising transplant outcomes and immunologic reconstitution.
Disclosures
No relevant conflicts of interest to declare.