Background
Relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a serious life-threatening complication. The granulocyte colony-stimulated factor mobilized donor lymphocyte infusions (gDLI) combined with chemotherapy is currently a commonly used treatment method. Nevertheless, gDLI may cause so severe acute graft-versus-host disease (aGVHD) as to impact prognosis. Posttransplant cyclophosphamide (PTCy) has been the backbone for GVHD prophylaxis by inducing tolerance to minor histocompatibility antigens in recipients, while the application of post-gDLI low-dose cyclophosphamide (PDCy) for GVHD prophylaxis has not yet been attempted.
Methods
To explore this possibility, a retrospective study was conducted. 20 patients relapsing after HSCT were administered 20 mg/kg/d cyclophosphamide(Cy)on day 3 (for matched related transplantation) or on days 3 and 4 (for haplo-identical or unrelated transplantation) after gDLI to prevent aGVHD (the PDCy group). Furthermore, through propensity score matching, 58 matched controls received other (for HID and URD) or no (for MSD) immunosuppressive therapy for GVHD prophylaxis (the Non-Cy group). Patients from the PDCy and the Non-Cy group were matched by transplantation type, CD3+ T cell counts, and age and gender of recipients and donors. An MRD complete response was undetectable MRD lower than 0.01% with qPCR, and a reduction of at least one-log fold in MRD load post gDLI was defined as an MRD response.
Results
The PDCy group comprised 9 and 11 men and women, respectively, with a median age of 38 (34-52, IQR) years. The Non-Cy group comprised 34 and 24 men and women, respectively, with a median age of 40 (29-47, IQR) years. The infused CD3+T cell dose in the PDCy and Non-Cy group was 3.0 *10^7/kg and 2.9 *10^7/kg, respectively (p=1.0). The interval between HSCT and gDLI was longer in the PDCy group when compared to the Non-Cy group (30.8 vs 6.5 months, p<0.01). And first recurrence accounted for a higher percentage in the Non-Cy group (93% vs 45%, p<0.01). Other transplant- and DLI-specific data did not exhibit any significant difference between the groups.
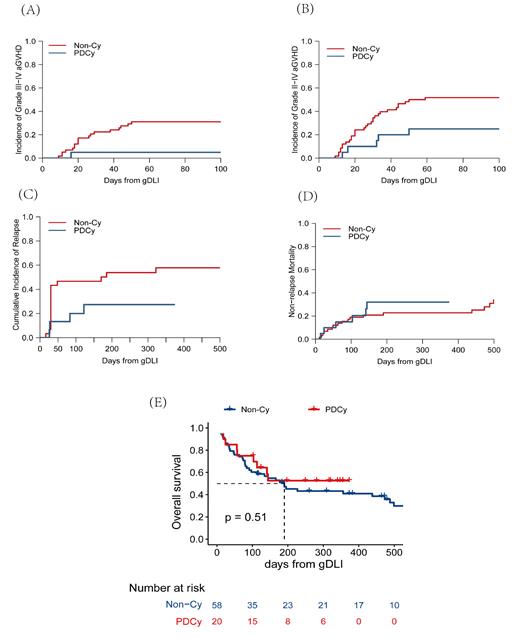
With a median follow-up of 4.8 (0-37.1) months, the PDCy group had lower cumulative incidence of severe aGVHD (III-IV, 5% vs 31%, p=0.02; II-IV, 25% vs 52%, p=0.04, figure 1A, B), but no significant differences existed in 4-month OS (64% vs 59%, p=0.51, figure 1E) and NRM (20% vs 19%, p=0.5, figure 1D). Among 49 patients undergoing salvage treatment (PDCy group, n=16; Non-Cy group, n=33), there was no statistical difference in 4-month CIR (20% vs 47%, p=0.11, figure 1C) and rates of objective response (PR+CR, 68.8% vs 54.5%, p=0.6). Among 29 patients undergoing preemptive treatment (PDCy group, n=4; Non-Cy group, n=25), there was no statistical difference in the rates of MRD complete response (25% vs 42%, p=1) and MRD response (25% vs 50%, p=0.6) between the PDCy group and the Non-Cy group. The PDCy regimen didn't increase the incidence of severe bacterial infection (45% vs 33%, p=0.3), bacteremia (25% vs 16%, p=0.11), fungus infection (20% vs 29.3%, p=0.4), virus infection (5% vs 19%, p=0.1), hemorrhagic cystitis (n=1 vs n=1), and cardiac events (n=0 vs n=0).
Conclusions
In conclusion, on the premise of safety, the PDCy regimen could effectively protest against severe aGVHD after gDLI while preserving therapeutic response rates. However, the research results still require verification through longer follow-up and large prospective randomized studies.
Disclosures
No relevant conflicts of interest to declare.