Background : Double-unit cord blood transplantation (dCBT) is standard therapy for patients with acute myelogenous leukemia (AML) who do not have suitable adult donors. We & others have reported low relapse rates & high progression free survival (PFS) in dCBT recipients suggesting robust CB-derived graft-versus-leukemia effects. However, the efficacy of dCBT in high-risk AML as determined by ELN genetic risk & pre-transplant minimal residual disease (MRD) status has not been performed to date.
Methods : All consecutive adult patients with AML who received first dCBT with intermediate intensity conditioning (Cy50/ Flu150/ Thio10/ TBI400) between 2014-2022 were analyzed. GVHD prophylaxis was with either Cyclosporine-A (CSA)/ Mycophenolate Mofetil (MMF) or CSA/MMF/Tocilizumab on an institutional protocol.
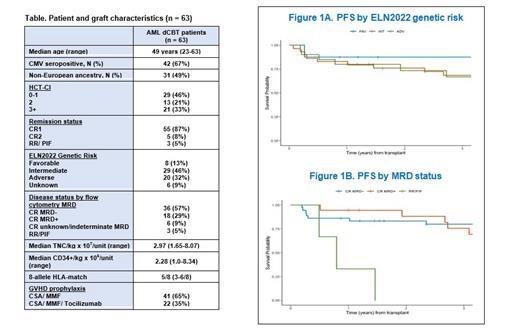
Results : 63 patients (pts) (median age 49 years, range 23-63) were eligible for analysis (Table); 42 (67%) were CMV seropositive, 31 (49%) had non-European ancestry, & median HCT-CI was 2 (range 0-6). Most (n = 55, 87%) were in CR1, 5 (8%) in CR2, & 3 were relapsed or primary refractory at transplant (5-9% blasts). By ELN2022 genetic risk criteria, 8 pts had favorable, 29 intermediate, & 20 adverse risk AML (6 were missing ELN status). Of the 60 pts in CR, 36 were MRD- & 18 MRD+ by multicolor flow cytometry (6 had unknown/ indeterminate MRD status).
Sixty-one of 63 pts engrafted for a 60-day cumulative incidence of 98% (95%CI:81-100). The median follow-up of survivors was 58 months (range 8-104 months). The 1- & 3-yr incidences of transplant-related mortality (TRM) were 13% (95%CI: 5.9-22) & 18% (95%CI: 9.6-29), respectively. The 1- & 3-year relapse incidences were 3.2% (95%CI: 0.6-9.9) & 8.6% (95%CI: 3.1-18), respectively. The 1- & 3-year OS were 87% (95%CI: 79-96) & 82% (95%CI:72-92) respectively, whereas the 1- & 3-year PFS were 84% (95%CI: 76-94) & 73% (95%CI: 63-85), respectively.
When stratified by ELN2022 genetic risk, the 3-year relapse incidence was 0% in favorable, 10% (95%CI: 2.5-25) in intermediate & 13% (95%CI: 2-36%) in adverse risk AML patients. Pts with favorable risk AML had a 3-year PFS of 88% (95%CI: 67-100); the 3-year PFS was similar in patients with intermediate [68% (95%CI: 53-88)] or adverse [67% (95%CI: 48-93)] risk AML (Figure 1A).
When stratified by MRD status, the 3-year relapse incidence was 8.4% (95%CI:2.1-20) in MRD- & 13% (95%CI: 1.9-34) in MRD+ patients. The 3-year PFS was similar in patients with AML in CR who were MRD- 80% (95%CI: 68-94) or MRD+ 76% (95%CI: 57-100), whereas all 3 relapsed/ refractory patients died of TRM (Figure 1B). Of 4 TP53-mutated AML patients, one relapsed, one died of TRM, & two are alive in remission.
Conclusions: While retrospective, our findings highlight the low relapse rates & promising PFS in adult AML patients, including those with high risk features such as adverse-risk ELN genetic risk &/ or MRD positivity supporting robust CB-derived graft-versus-leukemia (GVL) effects. This observation, together with the mitigation of TRM in recent years due to the optimized transplant practices in adult dCBT (see Politikos et al, ASH 2023), makes intermediate intensity dCBT an attractive strategy for AML patients with a suitable dCB graft & high-risk disease. Our findings warrant prospective multi-center investigation with correlative studies to elucidate the biology of CB-derived GVL.
Disclosures
Politikos:ExcellThera: Other: Membership on Data and Safety Monitoring Board ; Merck: Research Funding. Gyurkocza:Actinium Pharmaceuticals, Inc: Research Funding. Ponce:Kadmon/Sanofi Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ceramedix: Membership on an entity's Board of Directors or advisory committees; Evive Biotechnology: Membership on an entity's Board of Directors or advisory committees. Perales:MorphoSys: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Cidara Therapeutics: Consultancy, Other; Merck: Consultancy, Honoraria; VectivBio AG: Consultancy, Honoraria; Adicet: Honoraria; Medigene: Consultancy, Other; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Sellas Life Sciences: Consultancy; Syncopation: Honoraria; Karyopharm: Consultancy, Honoraria; Exevir: Consultancy, Honoraria; Caribou: Consultancy, Honoraria; Allogene: Research Funding; Celgene: Honoraria; Vor Biopharma: Consultancy, Honoraria; NexImmune: Consultancy, Current equity holder in publicly-traded company; DSMB: Other; Equillium: Consultancy, Honoraria; Allovir: Consultancy; AbbVie: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Servier: Other; Miltenyi Biotec: Honoraria; Incyte: Consultancy, Honoraria, Research Funding; Kite: Consultancy, Honoraria, Research Funding; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria. Barker:Gamida Cell: Consultancy; New York Blood Center: Consultancy; Merck: Research Funding.