Introduction:
Liver-targeted recombinant adeno-associated virus (rAAV) gene therapy for hemophilia B has recently become a real-world therapeutic option for an adult population burdened with prevalent co-morbid chronic hepatitis C virus (HCV) and hepatitis B virus (HBV). The pivotal phase 3 HOPE-B trial (NCT03489291) evaluated the efficacy and safety of etranacogene dezaparvovec (CSL222, formerly AMT-061), an AAV5 vector, containing a codon-optimized, highly active factor IX (FIX) Padua R338L transgene under the control of the liver-specific promoter LP-1. Here we evaluate the efficacy and safety of etranacogene dezaparvovec in the subset of HOPE-B participants with a history of chronic HCV and/or HBV.
Methods:
Adult male participants with hemophilia B (FIX ≤2%), were infused with a single dose of etranacogene dezaparvovec (2x10 13 gc/kg), following a ≥6-month lead-in period receiving their usual FIX prophylaxis. Relevant exclusion criteria included baseline liver chemistries > 2X the upper limit of normal (ULN); active HCV (HCV RNA detectable), HBV (HBV DNA detectable or HBV sAg reactive) or uncontrolled HIV infection; or advanced liver fibrosis (FibroScan™ score of ≥9 kPa). Regular liver ultrasound screening, serum chemistries, and alphafetoprotein (AFP) were collected along with FIX expression and bleeding data. During the first months post-treatment, alanine aminotransferase (ALT) increase to 2X the subject's baseline or >ULN was treated with a per-protocol tapering course of oral corticosteroids.
Results:
Among 54 HOPE-B trial participants, 31 (57.4%) had history of co-morbid chronic HCV, without active disease and with undetectable HCV RNA. Of these 31 subjects, 7 had a history of chronic HBV infection without active disease (HBV DNA undetectable; HBV sAg neg). Two subjects were HCV/HIV co-infected (HIV DNA neg; CD4+ T-cell count >200). Two subjects were HBV+ (HBV env AB reactive, HBV sAg non-reactive, HBV DNA undetectable)/HCV-/HIV-.
The mean age in the HCV+ and/or HBV+ subgroup (HCBV n=33) was 50.0 years (range 31-75). All HCBV participants had central lab (CL) ALT <ULN (ULN=41 U/L) on the day of dosing except one subject who had a CL ALT of 48 U/L. The mean screening fibroscan (liver elastography) score for this subgroup was 5.2 kPa (range 2.8-8.0). In HCBV participants 16/33 (48.5%) had pre-existing AAV5 neutralizing antibodies (NAb), with titers ranging from 8.5-3232; all NAb+ HCBV patients had titer ≤678 except one participant who had a titer of 3212 and never demonstrated FIX expression; as a result his prophylactic FIX infusions were never discontinued and his efficacy outcomes reflect exogenous FIX therapy. One HCBV participant returned to continuous routine prophylaxis 30 months post treatment.
In the HOPE-B trial 11/54 (20.4%) of participants had 12 adverse events of ALT elevation of 2X above baseline or >ULN within 12 weeks after etranacogene dezaparvovec treatment, which triggered immunosuppressive therapy with corticosteroids in 9/54 (16.7%) participants. In the HCBV subgroup, 5/33 (15.2%) participants had ALT elevations of which 4/33 (12.1%) participants received corticosteroids. As reported previously (Schmidt M, et al. Blood Advances. 2023), one HCBV subject maintained normal AFP levels however per-protocol screening ultrasound at one year after gene therapy this participant developed a hepatocellular carcinoma (HCC); molecular characterization demonstrated the HCC was not related to etranacogene dezaparvovec treatment.
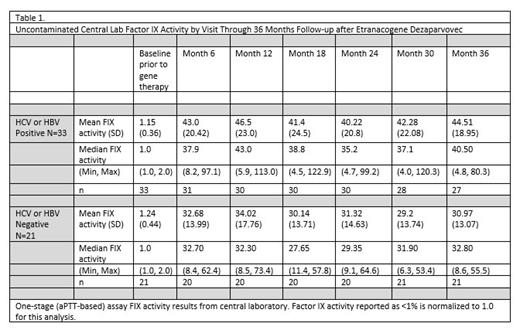
Factor IX expression was stably increased above baseline throughout 36 months after the single CSL222 infusion. History of chronic HCBV did not impact FIX expression (Table 1). After excluding the subject with the pre-existing AAV5 NAb titer 3212 whose continued prophylaxis use confounds analysis, the mean annualized bleeding rate (ABR) decreased after gene therapy compared to lead-in standard of care FIX prophylaxis. HCBV participants demonstrated an ABR rate ratio of 0.31 (95% CI 0.13, 0.72) indicating 69% reduction in all bleeding, sustained from months 7-36 following etranacogene dezaparvovec treatment.
Conclusions:
The majority of HOPE-B trial participants were adults with a history of chronic HCV and/or HBV infection without active viral disease or evident pre-existing liver fibrosis. Safety and efficacy are observed in the HCBV participants.
Disclosures
O'Connell:Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; F. Hoffmann-La Roche Ltd: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; UniQure: Membership on an entity's Board of Directors or advisory committees; Freeline: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Speakers Bureau. Verhamme:CSL Behring, Roche, CAP-DCF, Bayer HealthCare; LeoPharma; Boehringer Ingelheim; Daiichi Sankyo; Pfizer; Sanofi-Aventis; ThromboGenics: Consultancy. Meijer:Alexion and CSL Behring: Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; UniQure: Consultancy; Octapharma: Membership on an entity's Board of Directors or advisory committees. van der Valk:Bayer: Consultancy. Kazmi:BioMarin Pharmaceuticals, CSL Behring: Consultancy. Galante:CSL Behring: Current Employment. LeQuellec:CSL Behring: Current Employment. Church:CSL Behring: Current Employment. Lucas:CSL Behring: Current Employment. Castaman:CSL Behring, Pfizer, Sobi, Speaker Bureau of: Bayer, Biomarin, Roche, Sobi, Grifols, LFB, Novo Nordisk, Werfen, Kedrion, uniQure: Research Funding. Monahan:CSL Behring: Current Employment.