Introduction: Acquired hemophilia A (AHA) is a life-threatening bleeding disorder associated with significant morbidity and mortality. Despite publication of international consensus guidelines, quality of care and guideline adherence have not been examined. While some jurisdictions have reference centres for AHA management, in Canadian provinces outside of Quebec, AHA is managed in both hemophilia treatment centres (HTCs) and community hospitals. We performed a multicentre practice audit in a Canadian province to assess use of immunosuppressive therapy (IST), blood product utilization, and evaluate treatment burden and outcomes.
Methods: We included adults diagnosed with AHA (January 2000-December 2021) in Alberta, Canada, and examined the following quality of care indicators: delayed IST initiation from diagnosis, corticosteroid use alone in high-risk AHA (FVIII <0.01 IU/ml or FVIII inhibitor >20 BU/ml), cyclophosphamide use in low-risk patients (FVIII ≥0.01 IU/ml and inhibitor ≤20 BU/ml), prolonged cyclophosphamide use (>6 weeks), and inappropriate use of plasma, intravenous immunoglobulin (IVIG) and hemostatic products. Outcome measures included response, length of stay (LOS) and 30-day readmission. We evaluated treatment burden including frailty syndromes, increased level of care and hospitalization for treatment-related adverse events.
Results: Of the 38 patients diagnosed with AHA, 25 (66%) were female, the median age was 74 years (IQR 61-81), and median Charlson comorbidity index was 5 (IQR 3-7). Cardiovascular comorbidities were common, including venous thromboembolism (5; 13%), atrial fibrillation (5; 13%), and myocardial infarction (4; 11%). Fifteen (39%) had ≥1 frailty syndromes, including functional dependence (6; 16%), dementia (6; 16%), falls/fractures (5; 13%), and depression/anxiety (3; 8%). Five (13%) patients required higher levels of care (long term care placement) following discharge.
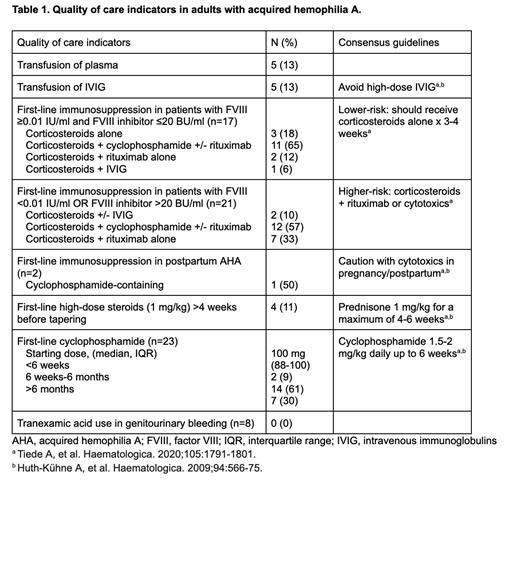
Twenty-one (55%) patients had high-risk disease and 17 (45%) had low-risk. In those with low-risk disease, 11 (29%) had inhibitor titres 5-20 BU/ml and 6 (16%) had inhibitors <5 BU/ml. Despite diagnostic delays ≥7 days from bleeding in 15 (39%), most received prompt initiation of IST (median 0 days [IQR 0-3] from diagnosis). First-line IST included: prednisone (38; 100%), cyclophosphamide (23; 61%), and rituximab (13; 34%). IVIG was prescribed in 5 (13%), out of keeping with guideline recommendations. We observed very high rates of first-line cyclophosphamide use in low risk AHA (11/17; 65%), including 4/6 with inhibitor titres <5 BU/ml (Table 1). First-line cyclophosphamide was also used in 3 reproductive-aged women. Prolonged courses of cyclophosphamide >6 weeks and >6 months were used in 21/23 (91%) and 7/23 (30%) patients, respectively. Two high-risk patients did not receive cyclophosphamide or rituximab-containing therapy.
Of the 58 episodes of bleeding events, 41 (71%) were ISTH major bleeds. Fifteen (26%) bleeding events did not receive hemostatic therapy, whereas 27 (47%), 19 (33%), and 3 (5%) were treated with recombinant factor VIIa, activated prothrombin complex concentrates, and porcine FVIII, respectively. Dosing of bypassing agents was in keeping with guidelines. DDAVP and human FVIII were inappropriately used in 3/58 (5%) and 1 (2%) bleeding episodes, respectively. Most patients (24; 63%) received red cell transfusions with a median of 2 (IQR 0-7) units each. Five (13%) patients received a total of 18 units of plasma.
Clinical responses included: complete remission (CR) in 30 (79%), partial remission in 3 (8%), and unevaluable due to early deaths in 5 (13%). All 5 patients who relapsed (13%) achieved second CR. The median LOS was 38 days (IQR 19-57) across 95 hospitalizations. The 30-day readmission rate was 24%. Reasons for admission included bleeding (50/95; 53%), infections (14/95; 15%), and other treatment side-effects (13/95; 14%).
Conclusion: In this provincial cohort of AHA patients with high comorbidity and frailty, we identified gaps in quality of care including overuse of cyclophosphamide in patients with low-titre inhibitors, prolonged duration of cyclophosphamide, and inappropriate use of IVIG and plasma. Aggressive cytotoxic therapy predisposes patients to unnecessary risks of infections and malignancies. Our findings highlight the need for centralization of care in specialized centres and education initiatives.
Disclosures
Goodyear:Sobi: Honoraria; Roche: Honoraria; Sanofi: Honoraria; Alexion: Honoraria; Takeda: Honoraria; BioCryst Pharmaceuticals: Honoraria; CSL Behring: Honoraria; Pfizer: Honoraria; Medison: Honoraria. Sun:Sobi: Honoraria; Takeda: Honoraria; Shire: Honoraria; Sanofi: Honoraria; Pfizer: Honoraria.