Background: Despite treatment safety and efficacy advancements for children with hemophilia (CwH), home treatment can be complicated and burdensome. Treatment burdens are major contributing factors to adherence issues.
The Child Hemo-TEM is a patient-centric outcome measure developed following the United States (US) Food and Drug Administration guidance for patient-reported outcome measure development. Designed to assess the treatment burden for CwH, the measure is a caregiver observer-reported outcome (ObsRO) measure where questions are answered by the caregiver about the child's treatment experience based on what the caregiver has seen, heard, or been told by the child.
Aim: The purpose of this study was to psychometrically validate the Child Hemo-TEM ObsRO.
Methods: Data was collected through a cross-sectional, web survey from a population of caregivers of CwH aged 2 to < 12 years in the US.
The survey consisted of the Child Hemo-TEM, sociodemographic items, child's hemophilia medical history, and supporting items/measures required to assess the measure's psychometric properties. The survey took up to 45 minutes to complete and was administered at a single time point.
To be eligible for the study, caregivers needed to be: aged ≥ 18 years; a caregiver of a male child with a diagnosis of severe to moderate hemophilia A or B, with a factor level of < 2%, with or without an inhibitor, and currently receiving either prophylactic or on-demand treatment; and living in the same residence as the child over the past 2 weeks. Recruitment was conducted by a patient advocacy group and a professional market research firm. Recruitment quotas were targeted to ensure a diverse population and mix of frequency of prophylactic treatment(s) and treatment administration type.
All psychometric analyses were conducted following an a-priori statistical analysis plan, which included examination of measurement characteristics, item reduction, factor analysis, measure scoring, internal consistency, reliability, and validity.
Results: Caregivers ( n=187) of CwH completed the web survey. The respondents were, on average, 37.8 years of age, mothers (84.0%), and predominantly White/Caucasian (74.3%).
Twelve items were dropped from the validation-ready, 19-item measure: 5 items due to high ceiling effects at baseline (>50%) mirrored by the Rasch results and 1 item as it was highly correlated with a retained item. Further, as there were only a small number of children who self-injected, 6 items designed to assess child self-injection were removed. The remaining items underwent further psychometric testing.
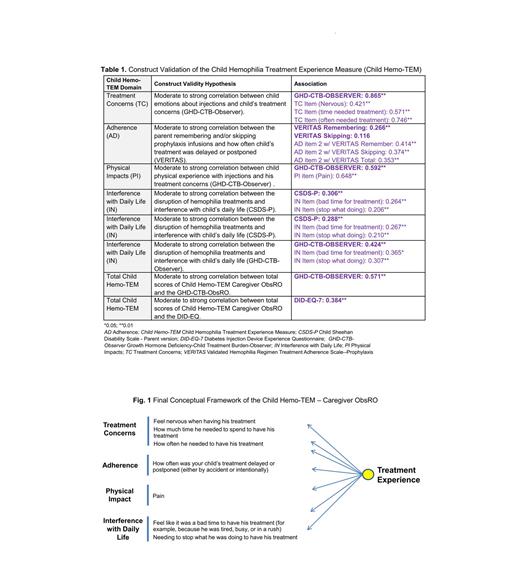
An exploratory factor analysis generated a single, 7-item factor. The Child Hemo-TEM was found to be internally consistent with a Cronbach's α of 0.855. A-priori validity hypotheses were adapted to match the final factor structure. Hypothesized associations were predominantly confirmed with a few exceptions. For convergent validity (Pearson's r significance set at α = 0.05), a few of the associations, although significant, were below 0.30 (Table 1). The Child Hemo-TEM was able to discriminate between levels of self-complaining about their infusions (p < 0.001), psychological stress reported by parent (p < 0.001), and ease/difficulty of their medication in its current form, among other variables.
Based on psychometric findings and taking into consideration learnings from the concept elicitation during measure development, the brief, 7-item Child Hemo-TEM was finalized with 1 domain capturing information from all the hypothesized concepts of treatment concerns (3 items), adherence (1 item), physical impact (1 item), and interference with daily life (2 items) (Figure 1). A five-point Likert response scale with ranges from “Never” to “Always”, or a “Don't know” option, is used.
Conclusion: The Child Hemo-TEM ObsRO has been found to be conceptually sound with adequate evidence to support its reliability and validity. Incorporation of the measure into both clinical and research settings will help assess the pediatric patient's experience of living with hemophilia as well as aid clinicians in tailoring treatments to patient characteristics and situations. Future studies are needed to assess test-retest reproducibility, sensitivity to change, and meaningful change thresholds of this new measure.
Disclosures
Brod:Novo Nordisk A/S: Consultancy. Busk:Novo Nordisk A/S: Current Employment, Current equity holder in publicly-traded company. Neergaard:Novo Nordisk A/S: Current Employment.