Introduction:
Patients with the most aggressive, hardest-to-treat, and rarer forms of multiple myeloma (MM), such as patients with severe renal impairment, central nervous system (CNS) disease, extramedullary disease, and plasma cell leukemia, have the greatest need of novel therapeutic approaches. Strict eligibility criteria for randomized controlled trials (RCTs) both deny access to clinical trials to these patients in greatest need, and also limit generalizability of trial results.
We thus systematically assessed newly diagnosed MM RCT's from 2006 to 2023 to ascertain the prevalence of restrictive eligibility criteria, and their trends over time.
Methods:
We searched MEDLINE/PubMed, Embase, and the Cochrane Registry of Controlled Trials to measure the prevalence of restrictive inclusion criteria MM RCTs. We included all published newly-diagnosed MM RCTs from 2006-2023. Restrictive eligibility criteria evaluated included 1) age exclusions, 2) projected life expectancy requirements, 3) performance status 4) requiring thresholds of “secretory disease”, 5) CNS disease, 6) plasma cell leukemia, 7) hepatic and/or 8) renal function requirements, 9) hematological thresholds, 10) prior malignancy, 11) history of neuropathy, and 12) exclusion of extramedullary disease. We searched manuscripts, clinicaltrials.gov listings, and individual protocols whenever publicly available to ascertain these criteria. We ascertained what proportion of trials had each of these twelve restrictive criteria and assessed trends over time. A logistical regression was used to compare these variables over time.
Results:
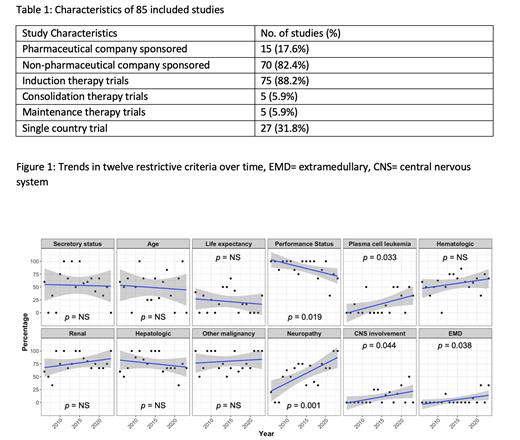
85 RCTs were included (Table 1). Exclusion criteria included: age in 43 (51%); projected life expectancy in 20 (24%); performance status in 74 (87%); thresholds of “secretory or measurable” disease in 47 (55%), , hepatic function in 64 (79%), renal function in 63 (74%), hematological thresholds in 50 (59%), prior malignancy (various time windows) in 68 (80%), neuropathy in 50 (59%) (various grades), plasma cell leukemia in 16 (18.8%), extramedullary disease in 4 (4.7%) and CNS disease in 9 (10.6%).
Trends in exclusion criteria were assessed over time (Figure 1) showing all criteria (other than performance status) have failed to improve from 2006 to 2023, and there has been an increase in exclusionary criteria pertaining to plasma cell leukemia, extramedullary disease, CNS involvement and pre-existing neuropathy.
Specific definitions of secretory disease varied with trials that required secretory disease (n=47), with 24 (51%) of these trials requiring a serum monoclonal protein of 1 g/dL or 0.5 if IgA/IgD/IgE/IgM MM, 2 (4.3%) requiring serum monoclonal protein of 2 g/dL regardless of MM subtype, 3 (6.4%) requiring serum monoclonal protein of 0.5 regardless of MM subtype, 11 (23.4%) requiring a serum free light chain of 10 mg/dL, and one (2.1%) requiring a serum free light chain of 5 mg/dL.
Conclusion:
The majority of MM RCTs have restrictive eligibility criteria that limit enrollment of the sickest MM patients and subsequent external validity of these trials, which perpetuates the status quo where limited data are available to guide the treatment of these challenging MM subtypes. It is very likely our methodology under-estimates the true prevalence of exclusionary criteria, as protocols were not universally available, and a trial was considered as having an exclusionary criteria only if explicitly listed. The presence of these restrictive eligibility criteria has failed to decrease over time.
Disclosures
Chakraborty:AbbVie: Research Funding; Genentech: Research Funding; Adaptive Biotech: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Costa:Genentech: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Adaptive biotechnologies: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; AbbVie: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Sborov:Abbvie: Consultancy; BMS: Consultancy; Pfizer: Consultancy, Research Funding; Bioline: Consultancy, Research Funding; Arcellx: Consultancy, Research Funding; Gilead: Research Funding; Amgen: Research Funding; Cantex: Research Funding; Sanofi: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; GSK: Consultancy, Research Funding; RocheX: Research Funding.