Introduction
Despite prophylactic platelet transfusions, World Health Organization (WHO) grade ≥ 2 bleeding occurs in 50 to 70% of patients with hematologic malignancies and hypoproliferative thrombocytopenia secondary to therapy and/or underlying disease (Gernsheimer et al, Blood 2022). Anemia (hematocrit, HCT ≤ 25%), coagulopathy (activated partial thromboplastin time ≥ 30s, international normalized ratio ≥ 1.2), and platelet count ≤ 5000/µl were independently associated with increased risk of bleeding in the PLADO (platelet dose) trial of 1272 patients with hypoproliferative thrombocytopenia treated between 2004 to 2007 (Uhl et al, Blood 2017). Updated data on bleeding outcomes and risk of bleeding is lacking.
Methods
We performed a post-hoc analysis of bleeding outcomes and bleeding risk factors in 330 patients with hematologic malignancy and hypoproliferative thrombocytopenia from therapy and/or underlying disease randomized at 3 institutions to receive either tranexamic acid (TXA) or placebo in the double-blinded, multicenter American Trial to Evaluate Tranexamic Acid Therapy in Thrombocytopenia (A-TREAT) (Gernsheimer et al, Blood 2022). Patients received prophylactic platelet transfusions for a platelet count of ≤ 10,000/µl and WHO grade bleeding was assessed daily for 30 days after a platelet count ≤ 30,000/µl and study drug was started. Study drug was discontinued when platelet count was ≥ 30,000/ul for 46 hours without platelet transfusion support or until a maximum of 30 days after treatment activation, whichever came first. Red blood cell (RBC) transfusion was given according to local standard of care. Cox proportional hazards regression with time-varying covariates was used to assess the association of patient characteristics with the risk of a first major (grade 2+) bleeding event. HCT, platelet count, inpatient vs. outpatient status, steroid treatment, and fever were recorded daily. Patients were censored at the first of time of withdrawal, death, or day 30.
Results
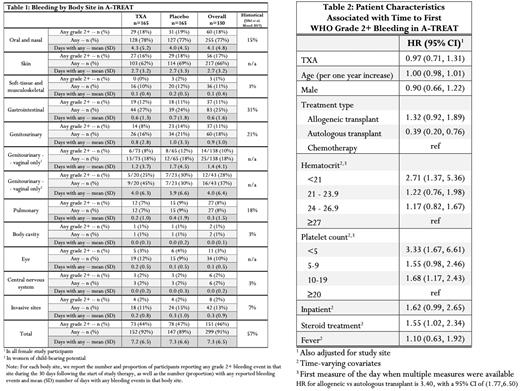
WHO grade ≥ 2 bleeding occurred in 46% of patients overall with no difference between the TXA and placebo groups (TXA 44% versus placebo 47%, p=0.66). Bleeding rates were highest in allogeneic stem cell transplant patients, followed by chemotherapy, followed by autologous stem cell transplant. Overall bleeding rates by organ system showed oral and nasal (18%), skin (17%), gastrointestinal (11%), and genitourinary (11%) bleeding (Table 1). WHO grade ≥ 2 vaginal bleeding occurred in 28% of patients of childbearing potential despite widespread use of hormonal suppression.
In a proportional hazards regression model of time to first bleeding event, undergoing allogeneic stem cell transplant was associated with a non-significant increased hazard of bleeding vs chemotherapy (HR 1.32, 95% CI 0.92-1.89), whereas autologous transplant had significantly lower hazard of bleeding vs chemotherapy (HR 0.39, 95% CI 0.20-0.76) (Table 2). HCT < 21% was associated with increased bleeding risk even after adjusting for severe thrombocytopenia (HR 2.71, 29% CI 1.37, 5.36). Platelet count < 5000/µl was also associated with increased risk (HR 3.33, 95% CI 1.67-6.61). Inpatient status, presence of fever, and age did not significantly impact risk of first bleed.
Conclusions
The overall rate of WHO grade >2 bleeding was similar to historical literature with lower rates of gastrointestinal bleeding than previously reported (31% in PLADO). Vaginal bleeding was common despite standard use of hormonal suppression in patients of childbearing potential. Platelet count < 5000/µl remains a risk factor for bleeding. Regardless of platelet count, bleeding risk increased with HCT < 21% suggesting a red blood cell transfusion threshold higher than 21% is unlikely to mitigate bleeding. More investigation is needed on strategies to reduce bleeding in this patient population.
OffLabel Disclosure:
Poston:TeraImmune: Consultancy. Jensen:Abbvie: Research Funding. Triulzi:Realta: Consultancy; Fresenius Kabi: Consultancy. Key:Novo Nordisk: Consultancy, Other: Chair of hemophilia grants study section; Biomarin: Consultancy, Ended employment in the past 24 months, Honoraria; Genentech: Consultancy; Uniqure/CSL: Consultancy, Ended employment in the past 24 months, Other: HOPE-B trial (hemophilia gene therapy) Steering Committee. May:Reata: Consultancy; Novo Nordisk: Consultancy; Global Health Labs (formerly Global Good): Consultancy; AtoxBio: Consultancy. Gernsheimer:Alpine Immune Science: Consultancy.
Tranexamic acid is an anti-fibrinolytic agent that is FDA approved for treating cyclic heavy menstrual bleeding. This study is a post-hoc analysis of bleeding outcomes of patients who were enrolled in a randomized control trial of tranexamic acid versus placebo.