Background:
Outcomes for patients with relapsed or refractory acute myeloid leukemia (R/R AML) remain extremely poor. Response rates with salvage chemotherapy regimens, such as mitoxantrone, etoposide, and Cytarabine (MEC), have been associated with a complete remission rate of around 20-30%. We previously reported a phase I study combining Lenalidomide (Len) with MEC salvage chemotherapy, with encouraging response rates in R/R AML compared to other historical standards (DeAngelo et al. AJH 2018). This led to the current phase 2 study (NCT03118466) of Len combined with MEC in R/R AML to evaluate for the efficacy of this combination.
Methods:
We conducted a phase 2 study of Len plus MEC in adult patients (≥ 18 yrs) with R/R AML after 1 or more cycles of induction chemotherapy. Patients received Len dosed at 50mg/day on days 1-10, and Mitoxantrone 8mg/m2/d, Etoposide 100mg/m2/d and Cytarabine 1000mg/m2/d on days 4 through 8 of induction (Kohrt et al AJH 2010). Patients who achieved a complete remission (CR), complete remission with incomplete blood count recovery (CRi) or incomplete platelet recovery (CRp) could proceed with consolidation chemotherapy or hematopoietic stem cell transplantation at the discretion of the treating physician. The study followed a Simon two-stage design powered to detect improvement to a 45% response rate. The primary objective was to determine the response rate. Secondary objectives included time to neutrophil and platelet count recovery, treatment related mortality, rates of relapse, and overall survival.
Results:
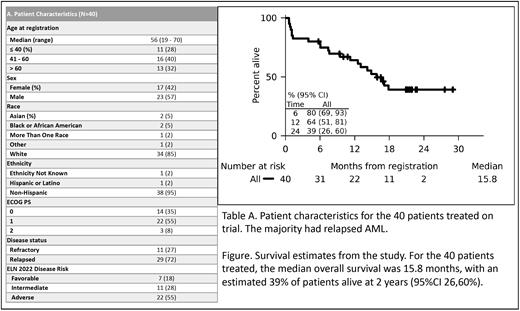
A total of 40 patients were enrolled and received study treatment. The median age was 56 years (range, 19-70); 58% of the patients were male. 73% of patients had relapsed after initial therapy (29/40) while 27% had primary refractory AML (11/40) after their initial induction chemotherapy. The median number of prior therapies was 2 (range, 1-4). The majority of patients had adverse ELN 2023 risk (n=22; 55%). Recurrent gene mutations in 5 or more patients included DNMT3A (n=8), TET2 (n=7), NRAS (n=6), IDH2 (n=5), and ASXL1 (n=5); 2 patients harbored TP53 mutations.
The most frequent grade 3-4 treatment-related toxicities were febrile neutropenia (n = 30; 75%), platelet count decrease (n = 40; 100%), anemia (n = 18; 45%), neutrophil count decrease (n = 14; 34%), blood bilirubin increase (n=6, 14%), and anorexia (n = 5; 12%). Three patients (8%) died during the study treatment period, all secondary to hepatic failure; no other early deaths occurred. Two of these patients had baseline grade 1 bilirubin elevation (no other patients in the study reported baseline bilirubin elevations).
Median follow-up was 21 months. The overall response rate was 50% (20/40) where 19 achieved CR while 1 achieved CRi. Of 20 responders, the median time to neutrophil count recovery was 28 days and to platelet recovery was 34 days. The median time to relapse was 22.6 months. The median overall survival (OS) of the entire cohort was 16 months (Figure 1).
Conclusions:
Len plus MEC was associated with a high response rate and improved OS in patients with R/R AML as compared to historical controls. The overall safety profile was similar to prior MEC chemotherapy trials but several patients did experience early hepatic toxicity. Overall these data support evaluating high-dose Len combined with MEC for patients with R/R AML in a randomized study.
Disclosures
Garcia:AbbVie: Consultancy, Research Funding; Astellas: Consultancy; Bristol Myers Squibb: Consultancy; Genentech: Consultancy, Research Funding; Gilead: Consultancy; Servier: Consultancy; New Wave: Research Funding; Prelude: Research Funding; Pfizer: Research Funding; AstraZeneca: Research Funding. Keng:Amgen: Research Funding. Hobbs:Protagonist: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Pharmaxis: Membership on an entity's Board of Directors or advisory committees; Keros: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Regeneron: Current holder of stock options in a privately-held company. Rosenblatt:Parexel: Consultancy; Bioclinica: Consultancy; Advare: Consultancy; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Other: Karyopharm; Sanofi: Research Funding; Bristol Myers Squibb: Research Funding. Luskin:Novartis: Honoraria, Research Funding; Pfizer: Honoraria; Jazz: Honoraria; AbbVie: Research Funding. Narayan:Sanofi: Other: Spouse employment ; Novartis: Other: Research funding to institution. Stone:Abbvie: Consultancy. Neuberg:Madrigal Pharmaceuticals: Current equity holder in private company. Avigan:Paraxel: Current Employment; Celgene: Consultancy, Other: Advisory role, Research Funding; Kite/Gilead: Consultancy, Other: Advisory role, Research Funding; Chugai Pharma: Consultancy, Other: Advisory role; Karyopharm Therapeutics: Consultancy, Other: Advisory role; Juno Therapeutics: Consultancy, Other: Advisory role; Legend Biotech: Consultancy, Other: Advisory role; Takeda: Consultancy, Other: Advisory role; Bristol-Myers Squibb: Consultancy, Other: Advisory board; Aviv Med Tech: Consultancy, Other: Advisory board; Partner Therapeutics: Consultancy, Other: Advisory board; Janssen: Consultancy, Other: Advisory board; Sanofi: Consultancy, Other: Advisory board; Kowa Pharmaceutical: Consultancy, Other: Advisory board; Pharmacyclics: Research Funding; Kite, a Gilead Company: Research Funding. Fathi:PureTech: Consultancy; Pfizer: Consultancy; Orum: Consultancy; Novartis: Consultancy; Menarini: Consultancy; Mablytics: Consultancy; Kite: Consultancy; Ipsen: Consultancy; Immunogen: Consultancy; Genentech: Consultancy; Forma: Consultancy; Daiichi Sankyo: Consultancy; Enclear: Consultancy; Celgene: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Autolus: Consultancy; Astellas: Consultancy; Amgen: Consultancy; Agios: Consultancy; AbbVie: Consultancy, Research Funding; Remix: Consultancy; Rigel: Consultancy; Servier: Consultancy, Research Funding; Takeda: Consultancy; Gilead: Consultancy. DeAngelo:Servier: Honoraria; Takeda: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Kite: Honoraria; Jazz: Honoraria; Incyte: Honoraria; Gilead: Honoraria; Blueprint: Honoraria; Autolus: Honoraria; Amgen: Honoraria; GlycoMimetics: Research Funding; Blueprint: Research Funding; Novartis: Research Funding; AbbVie: Research Funding. Brunner:Acceleron: Consultancy; AstraZeneca: Research Funding; Agios: Consultancy, Research Funding; Gilead: Consultancy; Takeda: Consultancy; Taiho: Consultancy; Novartis: Consultancy, Research Funding; Keros Therapeutics: Consultancy; Janssen: Research Funding; GSK: Research Funding; Celgene/BMS: Consultancy, Research Funding.