Objective: This research delineates the temporal alterations of cell-free circulating tumor DNA (ctDNA) in the plasma and cerebrospinal fluid (CSF) of patients harboring primary and secondary central nervous system (CNS) lymphoma. The aim is to establish that CSF serves as a superior reservoir of ctDNA compared to plasma, thereby aiding in diagnostic processes and monitoring therapeutic efficacy. Concurrently, this study underscores the enhanced response and survival rates in patients administered Bruton's tyrosine kinase (BTK) inhibitors over an extended period exceeding three months.
Methods: Data including cranial-enhanced magnetic resonance imaging (MRI) scans, leukocyte and protein concentrations in the CSF, exfoliated CSF cytology, flow cytometry, interleukin-10 to interleukin-6 ratios, ctDNA in plasma and CSF, were gathered from patients presenting primary and secondary CNS lymphoma(7 PCNSL,9 SCNSL). The temporal ctDNA modifications in plasma and CSF during the patient's disease progression-initial onset, remission, and relapse-were scrutinized. Additionally, the implications of BTK inhibitor usage on patient response rates and survival rates were assessed.
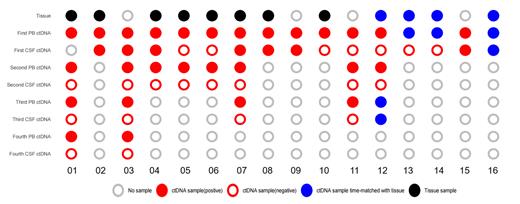
Results: Within the cohort of primary CNS lymphoma patients, ctDNA was detected in the CSF and plasma of 2 and 5 patients, respectively. Conversely, in patients with secondary CNS lymphoma, CSF and plasma ctDNA were discovered in 8 and 4 cases, respectively. The detection rate was contingent upon the central lesion's magnitude and position and the patient's surgical history related to the brain. Four patients, including two from each group, underwent cranial-enhanced MRI to appraise tumor burden at various disease stages, simultaneously with the monitoring of ctDNA in plasma and CSF. It was revealed that ctDNA levels in the CSF were in synchrony with the tumor burden, whereas plasma ctDNA levels remained relatively static. This indicates that CSF ctDNA may serve as a viable tool to assess therapeutic responses in CNS lymphoma patients. In two patients where surgical biopsy was unfeasible and both CSF exfoliation cytology and flow cytometry returned negative results, CSF ctDNA was positive, thereby underscoring its auxiliary role in diagnosing CNS lymphoma. Furthermore, the study indicated improved response and survival rates in patients subjected to BTK inhibitors for over three months.
Conclusion: CSF ctDNA demonstrates potential in detecting CNS lymphoma and in the dynamic monitoring of therapeutic responses. Compared to cranial-enhanced MRI, exfoliated CSF cytology, and flow cytometry, CSF ctDNA testing offers superior sensitivity. For patients for whom brain surgical biopsy is not an option, CSF ctDNA testing emerges as a valuable supplementary diagnostic tool. CSF ctDNA allows for dynamic monitoring of therapeutic responses in CNS lymphoma patients, enabling the detection of residual lesions, and thereby guiding the formulation and duration of the treatment regimen. Patients administered oral BTK inhibitors for more than three months demonstrate higher treatment response and survival rates.
Disclosures
No relevant conflicts of interest to declare.