Background: Mosunetuzumab is a CD20xCD3 bispecific antibody that is approved for the intravenous (IV) treatment of patients with relapsed/refractory (R/R) follicular lymphoma (FL) who have received at least two prior lines of systemic therapy, and is suitable for administration in the outpatient setting. In an ongoing Phase I/II study (NCT02500407), IV mosunetuzumab induced durable responses and had a manageable safety profile in patients with heavily pre-treated R/R indolent and aggressive B-cell non-Hodgkin lymphomas (NHL) when administered as a fixed-duration treatment (Budde et al. J Clin Oncol 2022). Subcutaneous (SC) dosing was also evaluated and showed similar efficacy to IV dosing and a manageable safety profile (Budde et al. ASH 2022). MorningSun (NCT05207670) is an ongoing, open-label, multicenter, Phase II study evaluating the efficacy, safety and pharmacokinetics of SC mosunetuzumab in patients with selected B-cell NHLs, including those without prior treatment (Flinn et al. ASCO 2022). For the first time, we present initial efficacy and safety data for patients with previously untreated, low-tumor burden FL who were enrolled into MorningSun at community and academic centers in the United States.
Methods: Inclusion criteria include previously untreated Grade (Gr) 1 or 2 FL, low tumor burden by GELF criteria, Ann Arbor stage III or IV disease, and ECOG performance status 0-2. Mosunetuzumab is administered without mandatory hospitalization by SC injection in 21-day cycles. In Cycle (C) 1, mosunetuzumab is given on Day (D) 1 (5mg), D8 (45mg) and D15 (45mg). In C2+, mosunetuzumab is given on D1 only (45mg). Treatment is continued for up to 17 cycles (~1 year). Patients who are in complete metabolic response (CMR) at C8 can discontinue active treatment and enter long-term follow-up. Premedication with dexamethasone (20mg) is mandatory in C1 and C2 and optional thereafter. Acetaminophen and diphenhydramine may also be given. The primary endpoint is the rate of progression-free survival at 24 months.
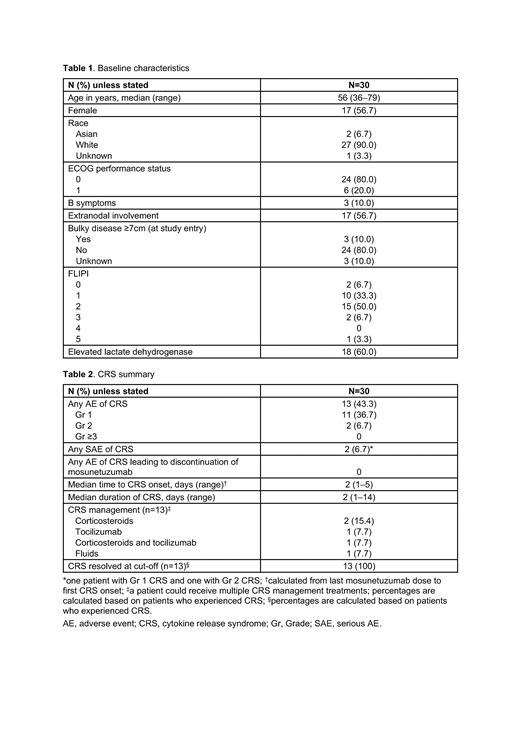
Results: At data cut-off (March 6, 2023), 30 patients had been enrolled (19 at community centers and 11 at academic centers). Median age was 56 years. All patients had ECOG performance status 0-1, and a minority had B symptoms ( Table 1).
The median duration of follow-up was 4.2 months (range: 0.2-11.1) and the median number of cycles received was 7 (range: 1-13). Among 24 patients who had at least one post-baseline tumor assessment, 23 achieved an objective response (95.8%; 95% CI: 78.9-99.9), including 20 who achieved a CMR (83.3%; 95% CI: 62.6-95.3). All responses were observed at the first post-baseline tumor assessment in C4 (median time to response: 2.6 months; 95% CI: 2.6-2.8). At cut-off, responses were ongoing in all patients who achieved CMR and 2 of 3 patients who achieved partial metabolic response.
The most common adverse event (AE) was injection site reaction (53.3% of patients), all of which were Gr 1 (43.3%) or Gr 2 (10.0%). Cytokine release syndrome (CRS) occurred in 43.3%. Most patients had Gr 1 CRS ( Table 2). No Gr ≥3 CRS events or discontinuations due to CRS were reported. CRS occurred in C1 only. All CRS events resolved. Other common (>20%) AEs were headache (50.0%), nausea (30.0%), fatigue (26.7%), pyrexia (23.3%), chills (23.3%) and alanine aminotransferase increased (23.3%). Gr ≥3 AEs in ≥5% of patients were neutrophil count decreased (2 patients; 6.7%). Serious AEs (SAEs) in ≥5% were CRS (2 patients; 6.7%). No SAEs of infection were reported. AEs potentially consistent with immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 2 patients (Gr 1 confusional state and Gr 1 memory impairment in one patient each); both events resolved. No mosunetuzumab-related AEs leading to discontinuation occurred and no Gr 5 (fatal) AEs were reported.
Conclusions: Initial MorningSun data support that SC mosunetuzumab is active in patients with previously untreated, low-tumor burden FL. Safety data demonstrate a manageable safety profile that is consistent with that seen in patients with R/R B-cell NHLs and is supportive of outpatient administration.
OffLabel Disclosure:
Flinn:Secura Bio: Consultancy; Novartis: Consultancy; Myeloid Therapeutics: Consultancy; Servier Pharma: Consultancy; Kite: Consultancy; Innocare Pharma: Consultancy; BeiGene: Consultancy; AbbVie: Consultancy; Genmab: Consultancy; Hutchinson MediPharma: Consultancy; Genentech: Consultancy; Century Therapeutics: Consultancy; TG Therapeutics: Consultancy; Vincerx Pharma: Consultancy. Budde:Amgen: Research Funding; AstraZeneca: Consultancy, Research Funding; Roche: Consultancy; Merck: Research Funding; ADC Therapeutics: Consultancy; MustangBio: Research Funding; Novartis, Gilead, F. Hoffmann-La Roche Ltd, BeiGene, Genentech, Inc.: Consultancy. Burke:Bayer HealthCare Pharmaceuticals: Consultancy; Verastem: Consultancy; Kymera: Consultancy; MorphoSys AG: Consultancy; Kura Oncology: Consultancy; BeiGene: Consultancy, Speakers Bureau; Epizyme: Consultancy; X4 Pharmaceuticals: Consultancy; Roche/Genentech: Consultancy; Bristol Myers Squibb: Consultancy; AstraZeneca: Consultancy; Adaptive Biotechnologies: Consultancy; Seagen Inc.: Consultancy, Speakers Bureau; Nurix: Consultancy; Morphosys: Research Funding; Gilead Sciences: Consultancy; AbbVie: Consultancy. Anz:Tennessee Oncology: Current Employment; Research is conducted through Sarah Cannon Research Institute with all payments going to SCRI and not individual physicians: Research Funding. Peles:Florida Cancer Specialists: Current Employment; American Oncology Network: Current equity holder in publicly-traded company; Florida Cancer Specialists: Current equity holder in private company; Sarah Cannon Research Institute: Research Funding; Karyopharm: Honoraria; Karyopharm: Speakers Bureau; Florida Cancer Specialists: Membership on an entity's Board of Directors or advisory committees. Sharman:Merck, Novartis: Consultancy; AbbVie, AstraZeneca, BMS, Beigene, Lilly, Genentech, Inc., Genmab: Consultancy; AbbVie, AstraZeneca, BeiGene, BMS, Genentech, Inc., Lilly: Consultancy; Seattle Genetics: Research Funding. Tumula:Texas Oncology: Current Employment. Biondo:F. Hoffmann-La Roche Ltd-: Current holder of stock options in a privately-held company; Genentech, Inc.: Current Employment; Genentech, Inc.: Ended employment in the past 24 months. Jani:Genentech, Inc.: Current Employment; Genentech, Inc. stock (RSUs): Current holder of stock options in a privately-held company; Genentech, Inc. (stock RSUs): Current equity holder in publicly-traded company. Wu:Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Lin:Genentech, Inc., GSK, AstraZeneca, Pfizer: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Parmar:Genentech, Inc.: Current Employment; Genentech, Inc.: Current holder of stock options in a privately-held company. Mun:FTE at Genentech, Inc.: Current Employment; Holds F. Hoffmann-La Roche Ltd SSARs, RSU, shares: Current equity holder in publicly-traded company. Yao:Genentech, Inc.: Current Employment.
Mosunetuzumab (Lunsumio) is a bispecific CD20-directed CD3 T-cell engager indicated for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy.