Introduction - Previously, our group reported results from ibrutinib with rituximab in older mantle cell lymphoma (MCL) patients (pts) with an atrial fibrillation rate of 22%. To develop a chemotherapy-free combination for elderly MCL pts, we designed this phase II clinical trial to investigate the efficacy and safety of acalabrutinib with rituximab (AR) as a first line approach in MCL pts (≥ 65 yrs).
Methods - We enrolled 50 previously untreated pts in this single institution, single arm, phase II clinical trial - NCT05214183. Pts received acalabrutinib 100 mg orally twice a day and intravenous rituximab, weekly for first 4 weeks, followed by once a month for 12 months and subsequently once every 2 months totaling 24 months. Acalabrutinib was continued. The primary objective was to assess best overall response rate (ORR) rate after AR. Responses were assessed as per Lugano response criteria. Clonoseq based minimal residual disease (MRD) assessment was performed. Adverse events were coded as per CTCAE version 5. Genomic studies are ongoing.
Results - Among 50 pts, the median age was 69 years (range: 65-81). Male/female were 36 and 14 respectively. Bone marrow was positive for MCL in 45/50 (90%) and GI tract in 35/47 (74%) pts. Forty-six pts had classic morphology while three were blastoid and one was pleomorphic. Ki-67% was available in 42 pts - 13/42 (31%) had high Ki-67% and 29/42 (69%) had a low Ki-67% (< 30%). Simplified MIPI risk stratification included: low (n=4), intermediate (n=35) and high risk (n=11). TP53 aberration status (mutations or deletion) was available in 43/50 pts and 12 pts had aberrant TP53 while 31/43 did not have aberrant TP53. Thirty-nine pts had baseline next generation sequencing (NGS) on tumor tissue biopsy and 25/39 (64%) had ≥ 3 mutations.
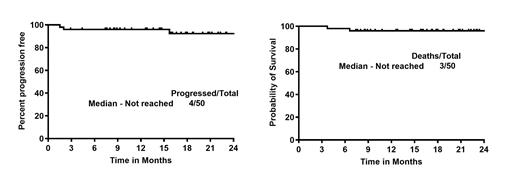
Median number of AR cycles was 12 (range: 3-24). One pt was not evaluable for response at 12 weeks. Overall, the best response was 94% ORR and 90% CR, 4% PR; 6% were non-responders. Median number of cycles to get complete metabolic response was 3 (range 2-7). Fourteen pts were MRD negative at last follow-up of the 28 evaluable pts.
With a median follow up of 17 months, the median PFS and OS were not reached (2 year 92% and 96% respectively). The median PFS and OS was not reached and not significantly different in pts with high and low Ki-67% or with/without TP53 aberrations or among pts with low, medium or high-risk categories. Nine pts (18%) came off study - 3 for adverse events (syncope, atrial fibrillation, intolerance), 3 for disease progression, one melanoma recurrence, one pt choice and one with an unusual monocytosis. Overall, 2 pts died (1 on trial with primary progression).
The most common all-grade toxicities were fatigue (82%), myalgia (64%), headache (38%), bruising (28%) and <1% were grade 3 or higher. Among 50 pts, 1 pt had recurrence of grade 2 atrial fibrillation (2%) and one pt had recurrence of grade 3 unstable angina.
Conclusions -Chemotherapy-free frontline therapy with AR is highly effective and safe and induced early CR in older pts with MCL.
Disclosures
Jain:AstraZeneca: Consultancy, Honoraria. Nastoupil:Gilead Sciences/Kite Pharma: Honoraria, Research Funding; AstraZeneca: Honoraria; Regeneron: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; DeNovo: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria; AbbVie: Honoraria. Westin:Kymera: Research Funding; AstraZeneca: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Calithera: Research Funding; Novartis: Consultancy, Research Funding; MonteRosa: Consultancy; Nurix: Consultancy; Morphosys/Incyte: Consultancy, Research Funding; SeaGen: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Abbvie: Consultancy; Genentech: Consultancy, Research Funding; Kite/Gilead: Consultancy, Research Funding. Iyer:Yingli: Consultancy, Research Funding; CRISPR: Consultancy, Research Funding; Innate: Research Funding; Acrotech: Consultancy, Research Funding; Legend: Research Funding; Astra Zeneca: Research Funding; Ono: Research Funding; Pfizer: Research Funding; Salarius: Consultancy; Drenbio: Research Funding; Merck: Research Funding; American Society of Transplant and Cellular Therapy: Speakers Bureau; CuraBio: Speakers Bureau; American Society of Hematology: Speakers Bureau; Seagen: Consultancy, Research Funding. Lee:Takeda: Research Funding; Pharmacyclics: Research Funding; Seagen Inc.: Research Funding; Aptitude Health: Honoraria; Olson Research: Honoraria; Korean Society of Cardiology: Honoraria; Deloitte: Honoraria; Curio Sciences: Honoraria; Oncternal Therapeutics: Research Funding; Century Therapeutics: Consultancy; Celgene: Research Funding; Cancer Experts: Honoraria; Janssen: Honoraria; Guidepoint: Honoraria; Bristol-Myers Squibb: Research Funding. Ahmed:Seagen: Research Funding; ADC Therapeutics: Consultancy; Servier: Consultancy; Kite/Gilead: Consultancy; Chimagen: Research Funding; Genmab: Research Funding; Merck: Research Funding; Tessa Therapeutics: Research Funding. Vega:Geron: Research Funding; Allogene: Research Funding. Wang:Bantam Pharmaceutical: Honoraria; CAHON: Honoraria; Dava Oncology: Honoraria, Other: Travel; VelosBio: Consultancy, Research Funding; Practice Point Communications (PPC): Honoraria; MJH Life Sciences: Honoraria; Studio ER Congressi: Honoraria; WebMD: Honoraria; Medscape: Honoraria; Scripps: Honoraria; Celgene: Other: Travel, Research Funding; Genentech: Research Funding; Moffit Cancer Center: Honoraria; Meeting Minds Experts: Honoraria; MD Education: Honoraria; NIH: Honoraria; IDEOlogy Health: Honoraria; i3Health: Honoraria; Genmab: Honoraria, Research Funding; Eastern Virginia Medical School: Honoraria; Juno Therapeutics: Research Funding; Loxo Oncology: Research Funding; Physicians Education Resources (PER): Honoraria, Other: Travel; Oncology Specialty Group: Honoraria; Nurix: Honoraria; OncLive: Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Pepromene Bio: Consultancy; Parexel: Consultancy; Oncternal: Consultancy, Research Funding; Milken Institute: Consultancy; Miltenyi Biomedicine: Consultancy; Merck: Consultancy, Honoraria; Eli Lilly and Company: Consultancy, Research Funding; Leukemia & Lymphoma Society: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria, Other: Travel, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; InnoCare: Consultancy; Genentech: Consultancy; DTRM Biopharma (Cayman) Limited: Consultancy; Deciphera: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria; BioInvent: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Be Biopharma: Consultancy; AstraZeneca: Consultancy, Honoraria, Other: Travel, Research Funding; Amphista Therapeutics Limited: Consultancy; ADC Therapeutics America: Consultancy; Acerta Pharma: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Molecular Templates: Research Funding; Vincerx: Research Funding. Flowers:Burroghs Wellcome Fund: Research Funding; Ziopharm: Research Funding; Xencor: Research Funding; TG Therapeutics: Research Funding; Takeda: Research Funding; Sanofi: Research Funding; Pharmacyclics: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Nektar: Research Funding; Morphosys: Research Funding; Kite: Research Funding; Jannsen Pharmaceuticals: Research Funding; Iovance: Research Funding; Guardant: Research Funding; Cellectis: Research Funding; Amgen: Research Funding; Allogene: Research Funding; Adaptimmune: Research Funding; Acerta: Research Funding; 4D: Research Funding; Spectrum: Consultancy; SeaGen: Consultancy; Pharmacyclics Jansen: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Karyopharm: Consultancy; Gilead: Consultancy, Research Funding; Genmab: Consultancy; Genentech Roche: Consultancy, Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Denovo Biopharma: Consultancy; Celgene: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Beigene: Consultancy; Abbvie: Consultancy, Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; Eastern Cooperative Oncology Group: Research Funding; V Foundation: Research Funding; National Cancer Institute: Research Funding; CPRIT Scholar in Cancer Research: Research Funding.