Introduction:
Plasmablastic lymphoma (PBL) is a rare and aggressive subtype of large B-cell lymphoma with plasmacytic differentiation. Although limited-stage (LS) PBL has a favorable outcome, extensive-stage (ES) PBL has a poor prognosis, with a median survival of 12-18 months. Patients with relapsed/refractory (r/r) PBL have limited treatment options and most die from the disease.
B cell maturation antigen (BCMA) - directed cellular and immunotherapies are highly effective in MM. The data on BCMA expression in PBL is limited. Literature review identified one report including 7 PBL cases all of which expressed BCMA. We studied all patients with a diagnosis of PBL who presented to Moffitt Cancer Center in Florida, USA from Jan. 2006 to Apr. 2023, and retrospectively performed BCMA immunohistochemistry (IHC) staining on the available archived FFPE biopsy samples.
Methods:
Progression-free survival (PFS) was calculated as time from disease progression or last follow up to treatment start date. Overall survival (OS) was calculated as time from death or last follow up to date of PBL diagnosis. Association of BCMA expression with PFS or OS was evaluated using logrank test, with continuous variables converted to categorical by classifying as above or below median. All analyses were done using SAS 9.2.
Results:
A total of 32 PBL patients were identified. The median age was 59 years (range 31-83 years); 21 (65.6%) had stage III/IV (ES); 9 patients (28.1%) were HIV positive; and 57.1% of evaluated cases were EBV positive by EBER. Treatment data was available for 30 patients. The most common frontline therapies were EPOCH and CHOP with or without rituximab; four patients received bortezomib combined with EPOCH; 7 patients (23.3%) received autoSCT in 1 st remission (Table 1). Median follow-up was 77.6 months calculated using the reverse Kaplan-Meier method. Consistent with previous reports, patients with ES PBL had worse outcome than those with LS PBL, with 3-year OS rates of 24% and 100%, respectively, p=0.01 (Table 1).
Among the 20 patients with ES PBL and known treatment responses, 11 did not achieve CR after frontline therapy. Ten of these 11 patients died due to disease progression, 9 within 2 years and 1 within 3 years. Two patients received CD19 CAR-T after multiple lines of therapy. Both died within 30 days, one from disease progression and the other from multiple treatment complications.
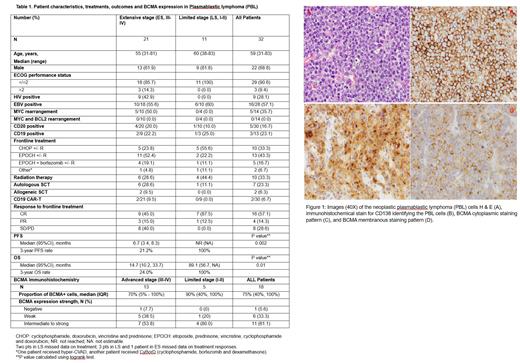
Out of the 18 cases with tissue available for IHC study, 17 (94.4%) had positive BCMA expression (Figure 1). The median percentage of BCMA+ cells was 75% (Table 1). Eleven (61.1%) cases had intermediate to strong BCMA staining. Neither BCMA+ cell percentage nor BCMA expression level was associated with disease stage, response rate, PFS, or OS (p>0.10 for all tests).
Discussion:
In our study the majority of our PBL cases tested positive for BCMA, and the percentage of BCMA+ cells seemed comparable to MM cases.To our knowledge, this is the largest and the first report that systemically evaluated BCMA expression in PBL. Our results provide evidence to explore BCMA as a potential target in PBL. Currently two anti-BCMA CAR-T products and an anti-BCMA/CD3 bispecific T-cell engager have been approved for r/r MM. A BCMA-targeting antibody-drug conjugate (ADC) Belantamab mafodotin-blmf was initially granted accelerated approval by FDA for r/r MM but was later voluntarily withdrawn from the market. Given the expression of BCMA in PBL and the lack of effective treatment of r/r PBL, these patients could be considered in trials evaluating the BCMA-targeting therapies. In fact, in a case report recently published, one patient with refractory PBL achieved CR after one infusion of BCMA CAR-T and remained in CR after 14 months. Ten of our 11 ES patients who did not achieve CR after frontline chemotherapy died from the disease, emphasizing the importance of clinical trials for this condition.
We did not find an association of BCMA expression with clinical outcomes as oppose of MM, in which higher BCMA expression was associated with shorter PFS and OS. The lack of association in our study may be due to the small sample size, or, it may reflect a difference in biological role of BCMA in PBL from MM.
Our results will need to be corroborated with other series to confirm our findings. Translational studies need to evaluate the dynamics of BCMA protein expression on PBL cell membrane and the activity of BCMA-targeting agents in vitro and in animal models of PBL.
Disclosures
Dong:Robert A. Winn Diversity in Clinical Trials Career Development Award, funded by Gilead Sciences: Research Funding; EUSA Pharma, a Recordati Group company.: Research Funding. Shah:Incyte, Jazz Pharmaceuticals, Kite/Gilead, SERVIER: Research Funding; Pharmacyclics/Janssen, Spectrum/Acrotech, BeiGene, Gilead Sciences: Honoraria; Celgene, Novartis, Pfizer, Janssen, Seattle Genetics, AstraZeneca, Stemline Therapeutics, Kite/Gilead: Other: Travel, Accommodations, Expenses; Takeda, AstraZeneca, Adaptive Biotechnologies, BMS/Celgene, Novartis, Pfizer, Amgen, Precision Biosciences, Kite/Gilead, Jazz Pharmaceuticals, Century Therapeutics, Deciphera, Autolus Therapeutics, Lilly, Pepromene: Consultancy; DSMC, Pepromene Bio: Membership on an entity's Board of Directors or advisory committees; Moffitt Cancer Center: Current Employment. Saeed:Epizyme: Consultancy; Novarits: Consultancy; Morphosis: Honoraria. Grajales-Cruz:Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Speakers Bureau; Cellectar: Membership on an entity's Board of Directors or advisory committees. Gaballa:Genentech: Consultancy; Ipsen: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Speakers Bureau; AstraZeneca: Consultancy; BeiGene: Consultancy; GiIead: Consultancy; TeneoBio: Research Funding; Elli Lilly: Speakers Bureau. Pinilla-Ibarz:Takeda: Consultancy, Speakers Bureau; Secura Bio: Consultancy, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau; Lilly: Consultancy, Speakers Bureau; Genentech: Speakers Bureau. Chavez:Karyopharm: Membership on an entity's Board of Directors or advisory committees; Genmab: Honoraria; Epizyme: Speakers Bureau; Cellectar: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra Zeneca: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Research Funding; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Lilly: Honoraria; Merck: Research Funding; Morphosys: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees.