Background: JAK2V617F (JAK2VF) is the primary driver mutation in classical myeloproliferative neoplasms (MPN). JAK2VF is present decades prior to diagnosis (Mitchell et.al. Nature 2022), and the JAK2VF+ clone may remodel the BM microenvironment further supporting its expansion (Curto-Garcia, et. al. Haematologica 2020). Understanding this relationship, particularly during early disease development may aid identification of new therapeutic targets to stop or revert these early changes. Human BM procured before MPN diagnosis is rarely available and most murine MPN models use myeloablative conditioning (eg., irradiation), permanently altering the BM stroma (Costa & Reagan Frontiers 2019), diminishing the clinical relevance of resultant findings. We developed an innovative translatable model of MPN with sustainable low-level disease engraftment and no requirement for recipient conditioning. By preserving the normal microenvironment, our model enabled detection of early changes within the BM stroma induced by the JAK2VF+ clone.
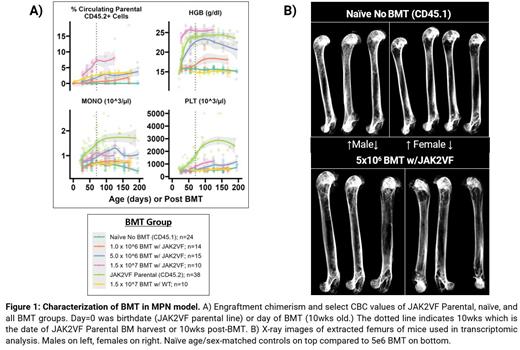
Methods: Whole BM transplantation (BMT) was performed via a single tail vein injection into unconditioned C57BL/6 Ptprc a (JAX#002014, CD45.1+) recipient mice. Donor animals consisted of either wildtype ( wt) C57BL/6 (JAX#000664) mice or our PolyI:C inducible MPN-like model created by crossing a floxed-JAK2VF (JAX#031658) with Mx1-Cre (JAX#003556) animals (JAK2VF Parental). For all BMTs, donors and recipients were age- (10wks) and sex-matched. BMT cell concentrations ranged from 1e6 to 15e6per injection, n≥10 mice, both sexes were used per group. A cohort of non-BMT age- and sex-matched recipients were maintained as naïve controls. Recipient serial submandibular blood collections were used to examine complete blood cell counts and donor chimerism. Bulk RNA-seq transcriptomic analysis was performed on digested and lineage-negative sorted whole BM from matched naïve and 5e6 BMT recipients (n=7 and 6, respectively) to delineate stromal alterations driven by JAK2VF+ MPN clone. Qualitative x-ray analysis was performed on femurs.
Results: 15e6 wt donor CD45.2 BM cells can be transplanted sustainably into unconditioned CD45.1 recipients with ~3.0% donor chimerism, maintained up to 200-days post-BMT (Fig A). Follow up secondary BMT into conditioned recipients using as few as 25e4CD45.2+ cells sorted from primary BMT recipients displayed 83% average engraftment by 150-day post BMT, confirming functional wt donor CD45.2 HSC. This successful strategy was used to perform BMT using JAK2VF parental (1e6, 5e6, and 15e6) cells. Detected chimerism followed a dose response, not exceeding an average of 10% up to >225 days. Surprisingly, with the engraftment at <10%, we observed MPN-like phenotype (elevated hemoglobin and monocytosis) except for no thrombocytosis (Fig A). To identify alterations in the BM niche, stromal cells were isolated from BMT recipients and subjected to RNA-seq. Enrichment of stromal cells was validated by confirming that 23% of the top 100 highest read coverage transcripts were encoding stromal genes (i.e. Sparc, Dcn, Col2a1, and Cxcl12). Gene set enrichment analysis (GSEA) of stromal fraction showed upregulation of osteoclastic differentiation and bone remodeling. GSEA against hallmark gene sets indicated upregulation of mesenchymal phenotype and inflammatory response suggesting an underlying effect on the stroma driven by the presence of the JAK2VF clone. Qualitative x-ray of femurs indicated loss of bone density, suggesting disease-driven effect on the stroma (Fig B).
Discussion: The development of our translatable model of MPN, where a small subset of cells drives the disease within unconditioned recipients represents an important milestone towards studying early disease pathology in MPNs. Using wt BMT, we demonstrated the successful establishment of long-term, low-level chimerism in unconditioned recipients. We found the clone-dependent impact on the stroma without myeloablative conditioning. Bulk transcriptomics and femur x-rays indicate aberrant bone morphology in BMT mice, despite the low level of engraftment (<5%) in the recipient system. Our data suggests that even low VAF of JAK2VF may support pathologic remodeling of the BM environment, warranting further exploration of patient BM niche early and late in the MPN course.
Disclosures
Reagan:Rigel: Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding.